Recombinant fusion alkaline phosphatase-allergen protein and preparation method and application thereof
An allergen protein and phosphatase technology, applied in biochemical equipment and methods, ovalbumin, transferrin, etc., can solve the problems of complex glycosylation modification function, inability to recombine protein modification, and difficult protein renaturation, etc. To achieve the effect of reducing non-specific reactions, improving detection sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
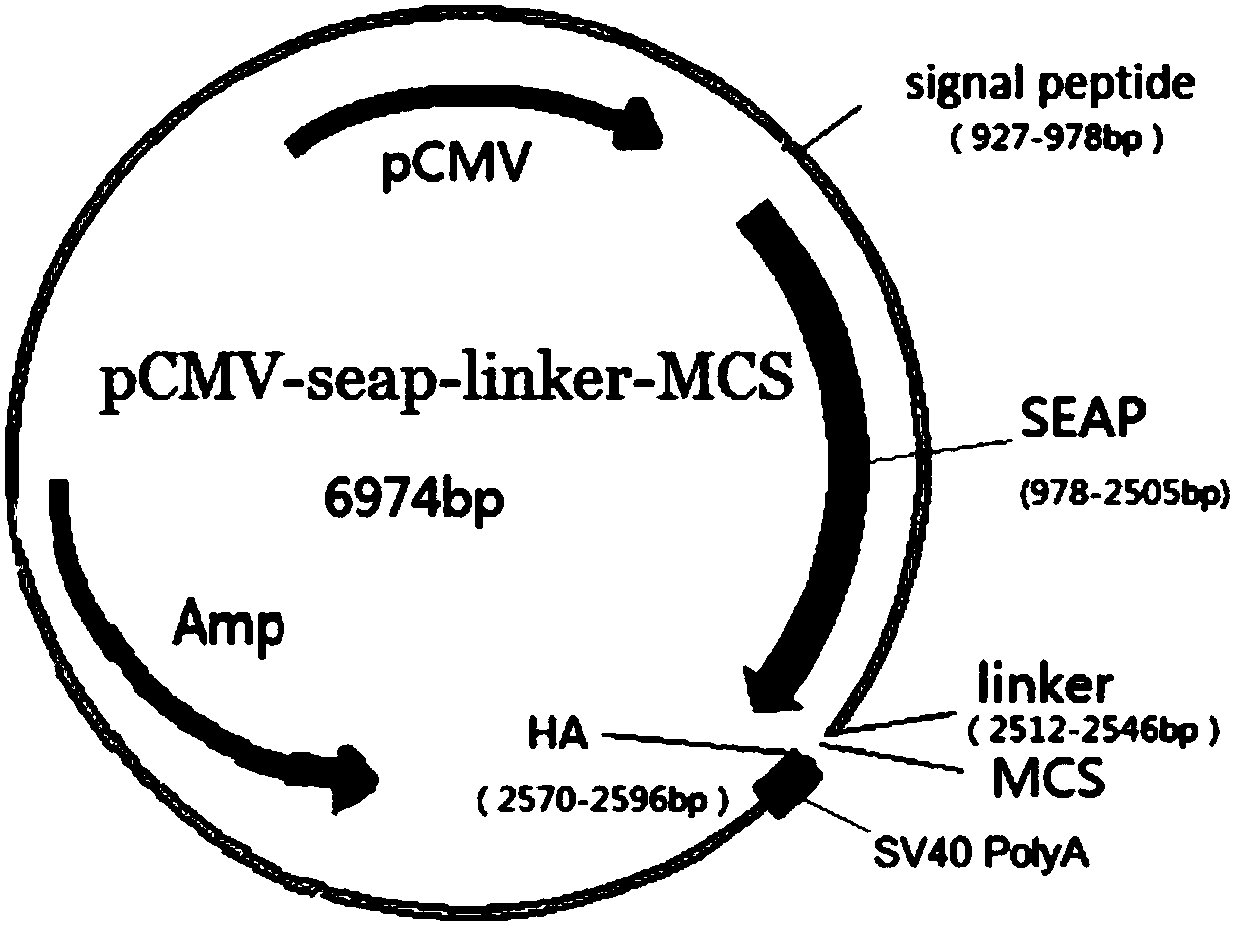
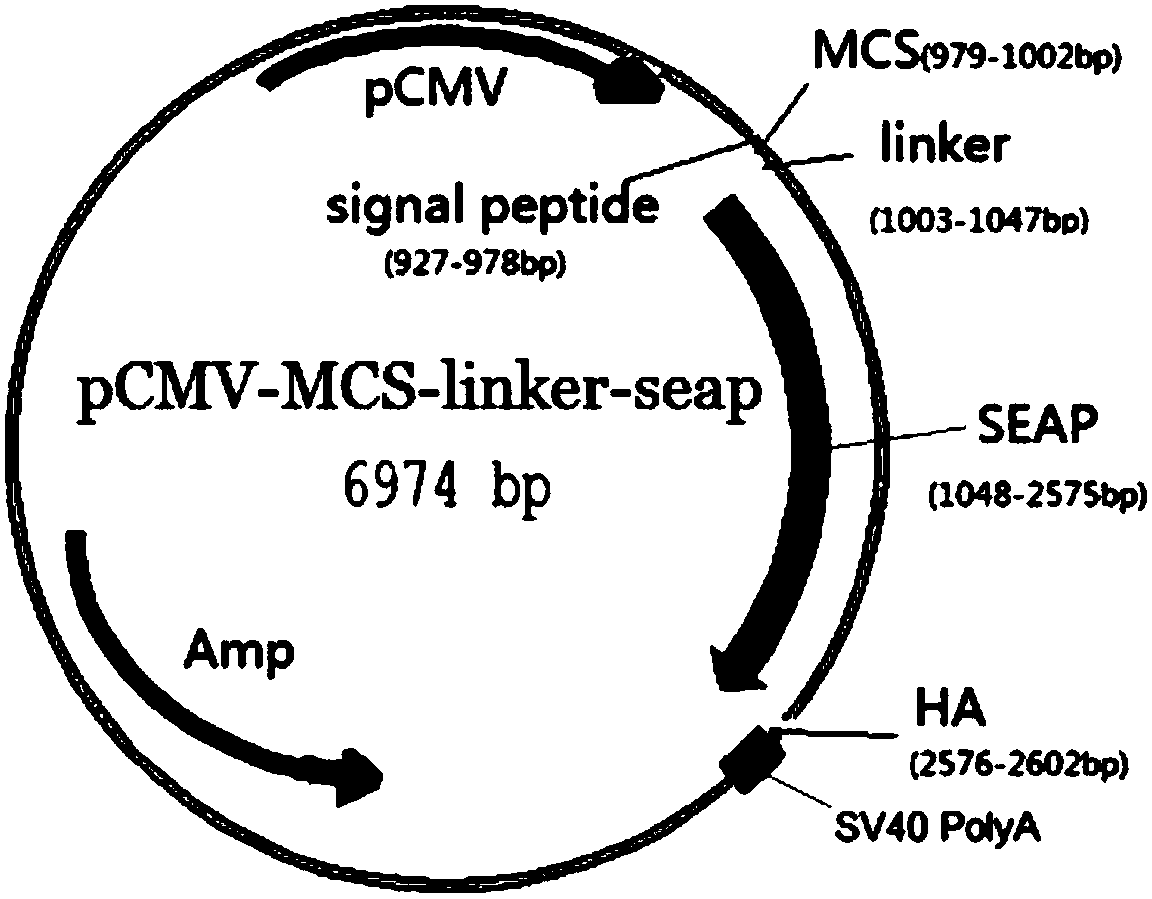
[0037] Example 1: Preparation of Alkaline Phosphatase Fusion Allergen Recombinant Plasmid
[0038] 1. Preparation method of allergen gene:
[0039] 1.1 Find the amino acid sequences of crustacean TM, crab CAK, egg Gald1, egg Gald2, egg Gald3, and cockroach Blag5 and Blag8 from the NCBI database, and use CODEHOP to design merger primers for each species.
[0040] 1.2 Tissue preparation
[0041] (1) Buy fresh shrimp from the vegetable market, cut the muscle into small pieces, and store them at -80°C for later use.
[0042] (2) Remove the shells of the bought live crabs, scrape the muscles with a knife, cut into small pieces, and freeze them at -80°C for later use.
[0043] (3) Purchase chicken oviducts freshly taken out of the chicken from the vegetable market, cut them into pieces with scissors, and store them frozen at -80°C for later use.
[0044] (4) German cockroaches caught indoors were starved overnight, cut into pieces, and frozen at -80°C for later use.
[0045] 1.3 G...
Embodiment 2
[0064] Example 2: Construction of CHO stable cell lines expressing allergens
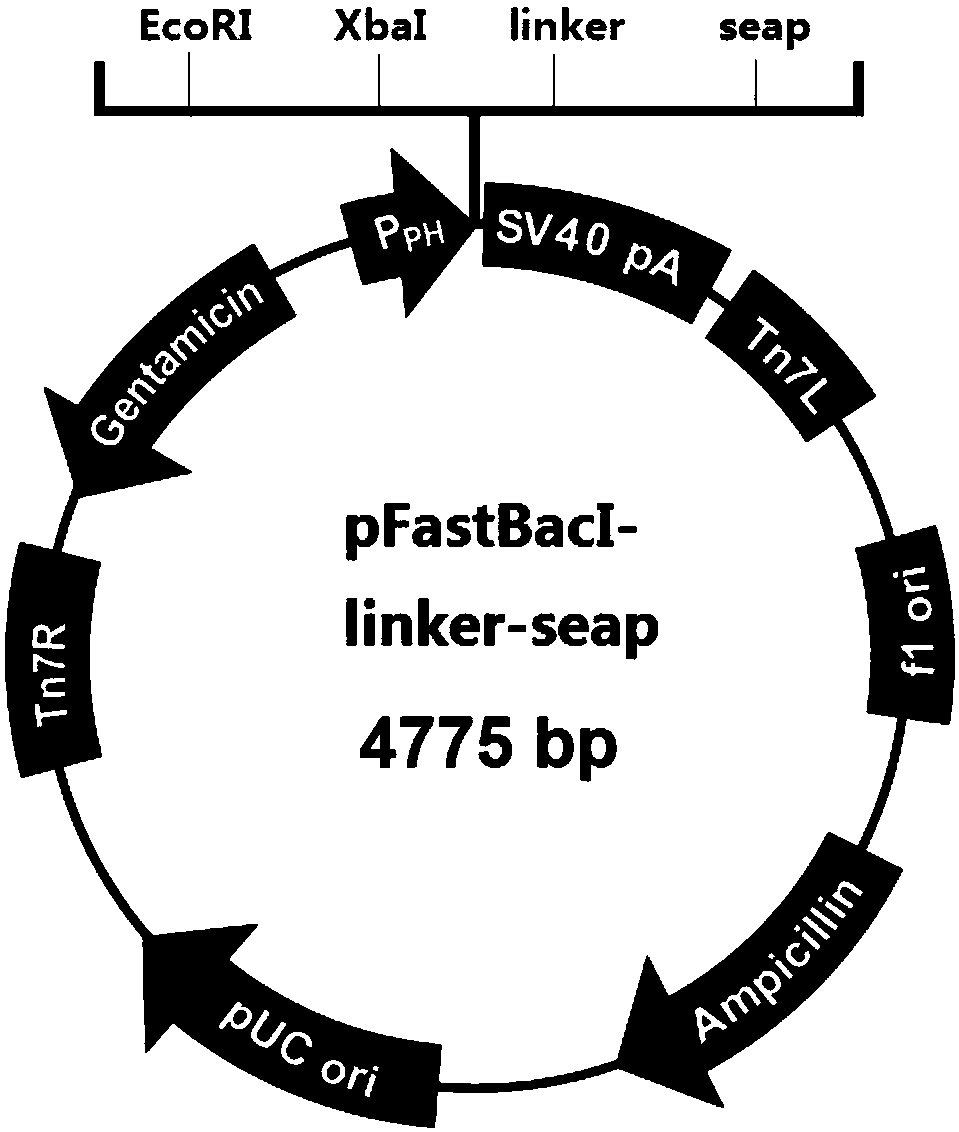
[0065] In this embodiment, the fusion expression of alkaline phosphatase-allergen is carried out in mammalian cell CHO. In other embodiments, host cells are selected from insect cells, yeast and plant cells. In this example, a CHO stable cell line expressing allergens was constructed.
[0066] 1. Culture medium, reagents, equipment, etc. used for cell line construction:
[0067] Cell: CHO;
[0068] Medium: CHO serum-free medium, fetal bovine serum, double antibody;
[0069] Freezing medium: fetal bovine serum or newborn bovine serum containing 10% DMSO
[0070] Antibiotics: Puromycin;
[0071] trypsin;
[0072] Transfection kit lipofectamine2000;
[0073] CO 2 incubator;
[0074] Ultra-clean workbench;
[0075] Microscope etc.
[0076] 2. Stable cell line construction steps
[0077] 2.1 Puromycin (puromycin) concentration screening
[0078] (1) Well-grown CHO cells are seeded into 24-wel...
Embodiment 3
[0099] Embodiment 3: Alkaline phosphatase fusion allergen recombinant protein production
[0100] Media, reagents, equipment, etc. used in the production of allergen recombinant proteins:
[0101] Medium: CHO serum-free medium;
[0102] Equipment: shaker bottle, shaker;
[0103] Allergen recombinant protein fermentation steps are as follows:
[0104] 1. Resuscitated and screened stable cell line CHO;
[0105] 2. Subculture to 4ml medium, grow for about 3 days, and identify by ELISA;
[0106] 3. Digest the cells, subculture to 10ml medium, culture for 2-3 days, add 10ml medium, make the cell density not exceed 1*10 6 a / ml;
[0107] 4. Subculture 20ml of cells into a 250ml large bottle, continue to cultivate, and add fresh medium continuously during the period, so that the cell density does not exceed 1*10 6 cells / ml, so that the volume of cell fluid does not exceed 1 / 3 of the bottle capacity;
[0108]5. Change the 500ml culture bottle and repeat 4.
[0109] 6. Collect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com