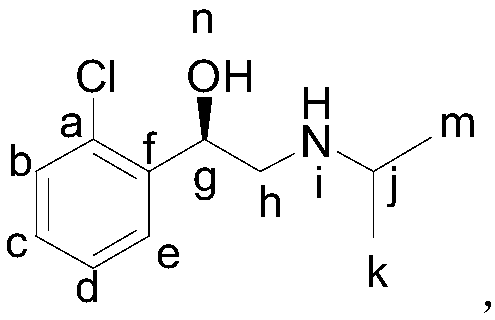
Stable isotope tag R-clorprenaline and preparation method thereof
A technology of stable isotope and chlorprenaline, which is applied in the field of stable isotope labeling R-chlorprenaline and its preparation, can solve the problems of no synthetic steps of chlorprenaline, long reaction route, cumbersome preparation, etc., and achieve economical Good performance and use value, high utilization rate of isotope atoms, and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
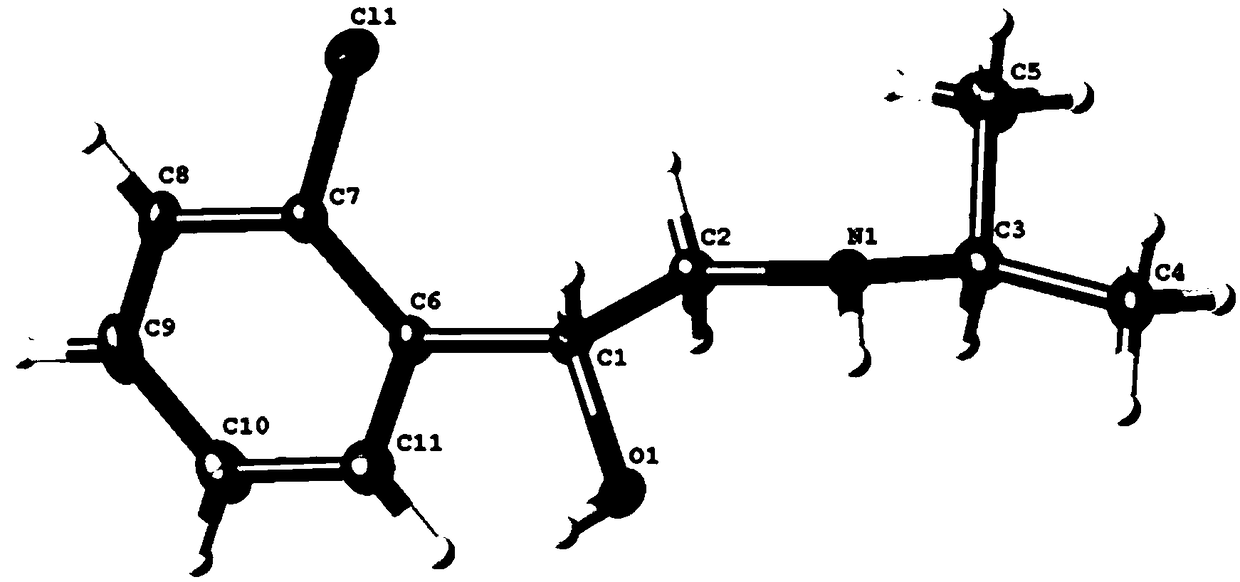
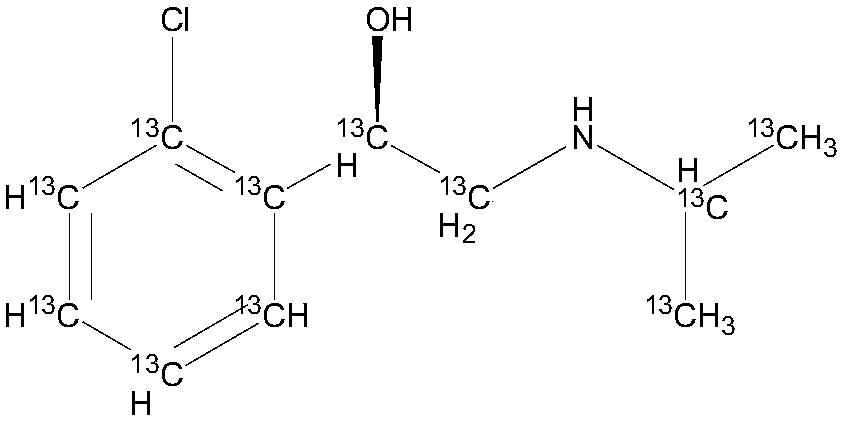
[0043] A stable isotope labeled R-chlorprenaline- 13 C 11 The preparation method comprises the following steps:
[0044] 1. Stable isotope labeled o-chloroacetophenone- 13 C 8 preparation of
[0045] Under anhydrous and anaerobic conditions, 11.9g chlorobenzene- 13 C 6Add it into a 250ml three-necked flask, add 25ml of tetrahydrofuran, 5g of titanium tetrachloride, stir and disperse, add dropwise acetic anhydride- 13 C 4 11.5g, after the dropwise addition, the temperature was raised to 80°C for 2 hours, washed with water, dried and recrystallized to obtain o-chloroacetophenone- 13 C 8 15.6g, yield 94.3%, purity 99.5%, abundance 99.5atom% 13 c.
[0046] 2. Stable isotope labeling α-bromo-o-chloroacetophenone- 13 C 8 preparation of
[0047] Under anhydrous and anaerobic conditions, add 8.1g o-chloroacetophenone to a 250mL three-necked flask- 13 C 6 , add 15g of DBBA, add 20ml each of methanol and ethyl acetate, control the temperature at 60°C, react for 4 hours,...
Embodiment 2
[0053] A stable isotope labeled R-chlorprenaline-D 16 The preparation method comprises the following steps:
[0054] 1. Stable isotope labeling o-chloroacetophenone-D 7 preparation of
[0055] Under anhydrous and anaerobic conditions, 11.7g of chlorobenzene-D 5 Add it into a 250ml three-necked flask, add 35ml of deuterated chloroform, 3g of zirconium tetrachloride, stir and disperse, add dropwise acetic anhydride-D 6 12.0g, after the dropwise addition, the temperature was raised to 60°C for 2 hours, washed with heavy water, dried and recrystallized to obtain o-chloroacetophenone-D 7 15.4g, yield 95.8%, purity 99.6%, abundance 99.0% atom%D.
[0056] 2. Stable isotope labeling of α-bromo-o-chloroacetophenone-D 7 preparation of
[0057] Under anhydrous and anaerobic conditions, add 8.1g o-chloroacetophenone-D to a 250mL three-necked flask 7 , add 14g of DBI, add 20ml of deuterated chloroform, control the temperature at 40°C, react for 3 hours, wash with water, dry and re...
Embodiment 3
[0063] A stable isotope labeled R-chlorprenaline- 15 N preparation method, the method comprises the following steps:
[0064] 1, the preparation of o-chloroacetophenone
[0065] Under anhydrous and anaerobic conditions, add 11.2g of chlorobenzene into a 250ml three-necked flask, add 35ml of dichloromethane, 2g of antimony trichloride, stir and disperse, add 12.9g of acetic anhydride dropwise, after the dropwise addition, raise the temperature React at 50°C for 1 hour, wash with water, dry and recrystallize to obtain 14.6 g of o-chloroacetophenone with a yield of 94.3% and a purity of 99.5%.
[0066] 2. Preparation of α-bromo-o-chloroacetophenone
[0067] Under anhydrous and anaerobic conditions, add 7.5g of o-chloroacetophenone, 10.1g of dibromohydantoin, and 20ml of methanol into a 250mL three-necked flask, and then add 20ml of methanol, keep the temperature at 40°C, react for 4 hours, wash with water, dry, and recrystallize Finally, 10.4 g of yellow solid was obtained, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com