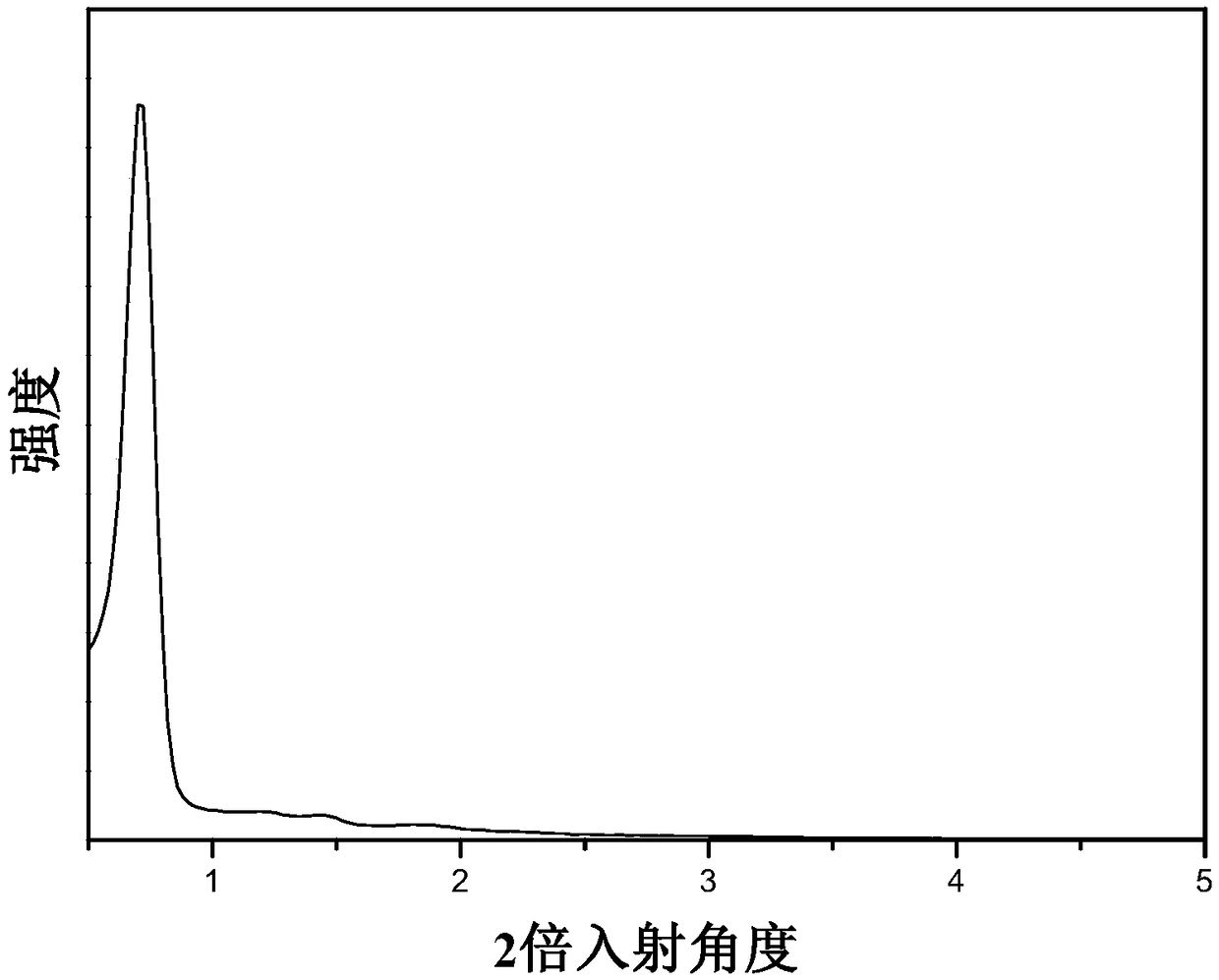
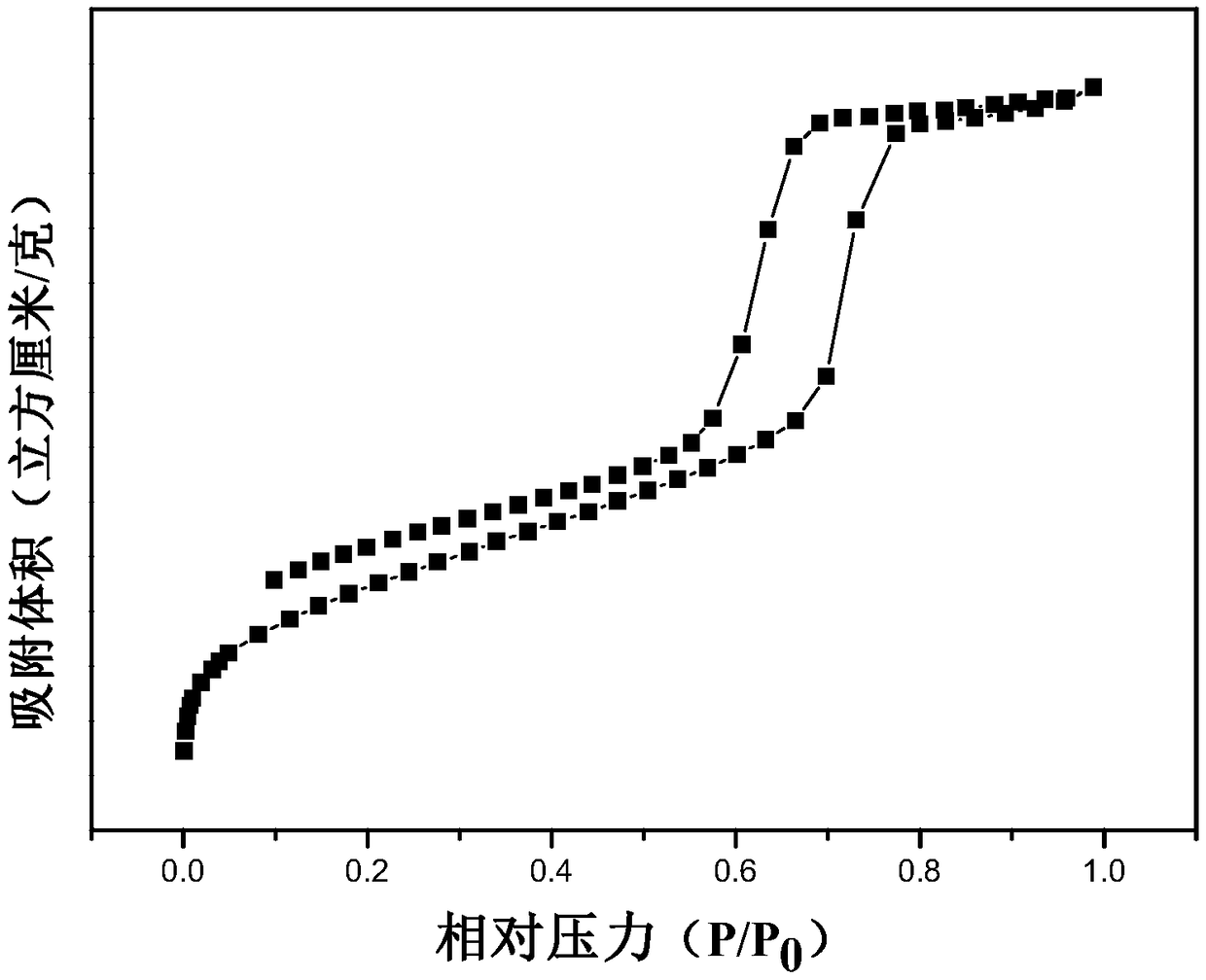
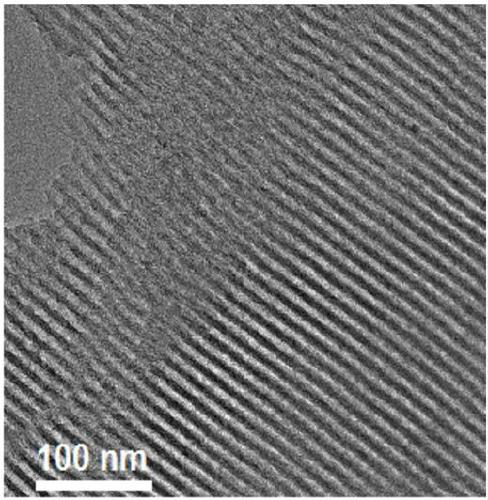
Preparation and application of bifunctional ionic liquid loaded mesoporous polymer
An ionic liquid and polymer technology, which is applied in the preparation of organic compounds, the preparation of carboxylic acid amides, organic compounds/hydrides/coordination complex catalysts, etc. problems, to achieve the effects of good thermal and chemical stability, high catalytic activity, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of imidazole-functionalized ordered mesoporous materials
[0037] A, the preparation of 1-(3-methoxybenzyl)-1-hydrogen-imidazole intermediate
[0038] After mixing 5 mL of tetrahydrofuran with a mass concentration of 99.9% and 4.3 g (0.18 mol) of sodium hydride, add dropwise to a mixture of 40 mL of tetrahydrofuran with a mass concentration of 99.9% and 6.8 g (0.1 mol) of imidazole, and stir and mix for 40 minutes Add 15.7g (0.1mol) 1-(chloromethyl)-3-anisole ether with a mass concentration of 99.9%, and reflux at a temperature of 70° C. for 24 hours. The reaction structure is as follows:
[0039]
[0040] The reaction product was washed three times with deionized water and extracted with dichloromethane. After extraction, the organic phase was washed twice with deionized water and once with saturated brine, then dried with anhydrous sodium sulfate, spin-dried with dichloromethane, and then subjected to column chromatography After separation, 15.3 g ...
Embodiment 2
[0060] Take by weighing 100mg (4mol%) 3-IMP-MPs-Et-(NEt) prepared in the above-mentioned embodiment 1 4 )AcO as a catalyst and 107mg (1mmol) nitrogen methyl aniline, 210mg (2mmol) phenylsilane and 2ml acetonitrile are put into the autoclave, and filled with carbon dioxide with a pressure of 1MPa, heated to 30°C, reacted for 18 hours, the reaction The structural formula is:
[0061]
[0062] CO during the reaction 2 The pressure was kept constant. After the reaction, the reactor was cooled to room temperature, and 20ml of deionized water was added to wash and quench the phenylsilane. The catalyst separated by suction filtration could be recycled. Add 60ml of dichloromethane to the filtrate and extract three times to take the organic layer, add anhydrous sodium sulfate to dry for half an hour after merging the organic layers, and the oily mixture obtained by rotary evaporation is separated by column chromatography (petroleum ether: ethyl acetate=4: 1), to obtain 128 mg of n...
Embodiment 3
[0064] Take by weighing 100mg (4mol%) the 3-IMP-MPs-Et-(NEt4)AcO that above-mentioned embodiment 1 prepares is catalyst and 121mg (1mmol) p-methyl nitrogen methyl aniline, 210mg (2mmol) phenylsilane and 2ml acetonitrile release Put it into a 15ml autoclave, and fill it with carbon dioxide with a pressure of 1MPa, heat it to 30°C, and react for 18 hours. The reaction structure formula is:
[0065]
[0066] CO during the reaction 2 The pressure was kept constant. After the reaction, the reactor was cooled to room temperature, and 20ml of deionized water was added to wash and quench the phenylsilane. The catalyst separated by suction filtration could be recycled. Add 60ml of dichloromethane to the filtrate and extract three times to take the organic layer, add anhydrous sodium sulfate to dry for half an hour after merging the organic layers, and the oily mixture obtained by rotary evaporation is separated by column chromatography (petroleum ether: ethyl acetate=4: 1), to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com