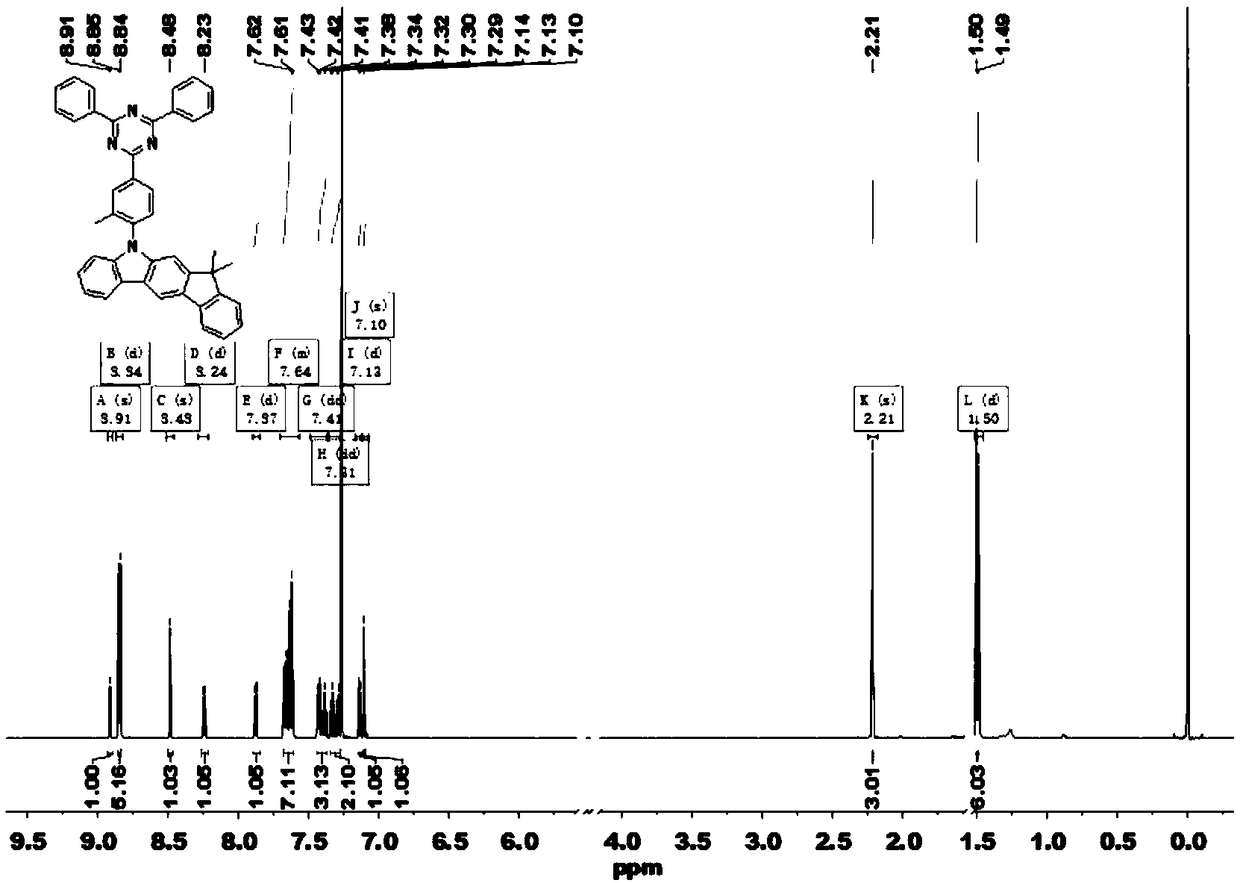
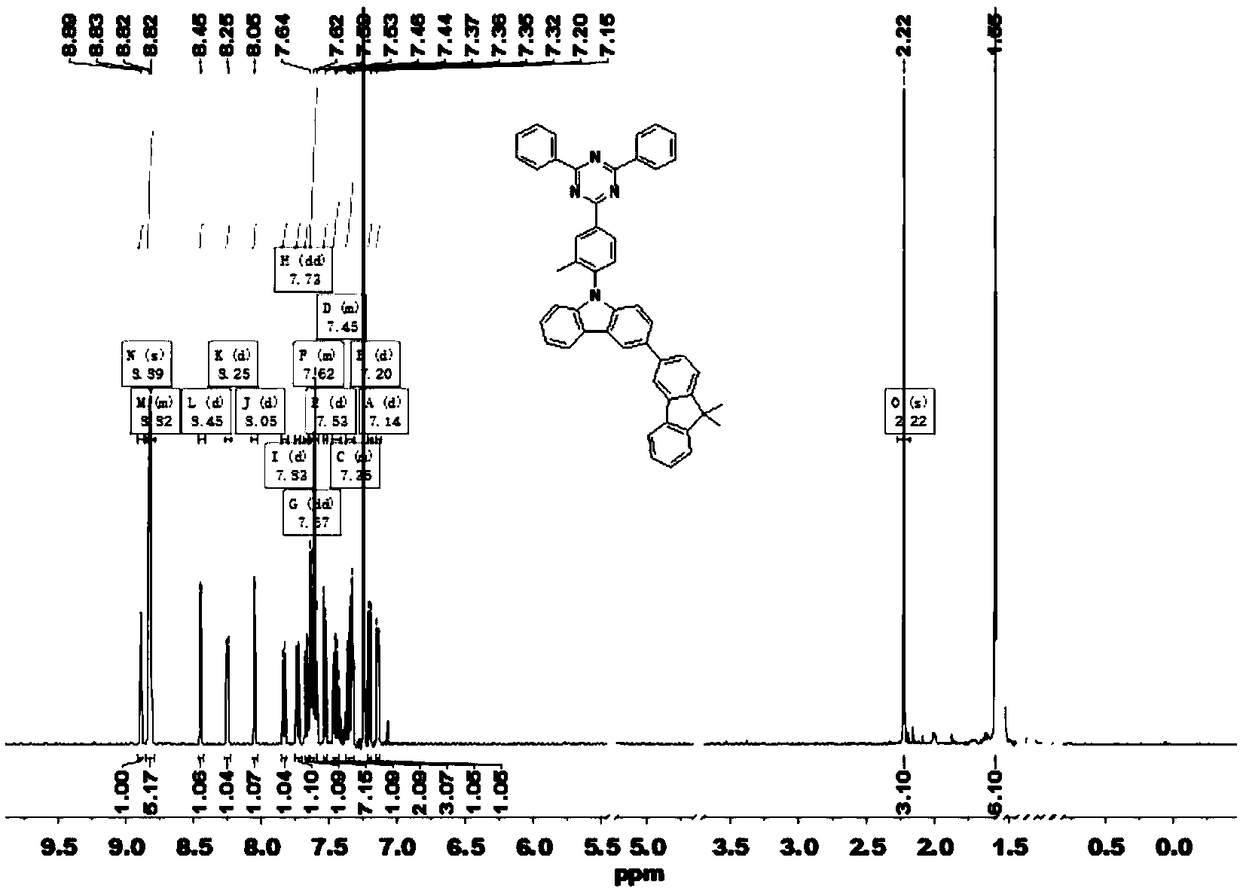
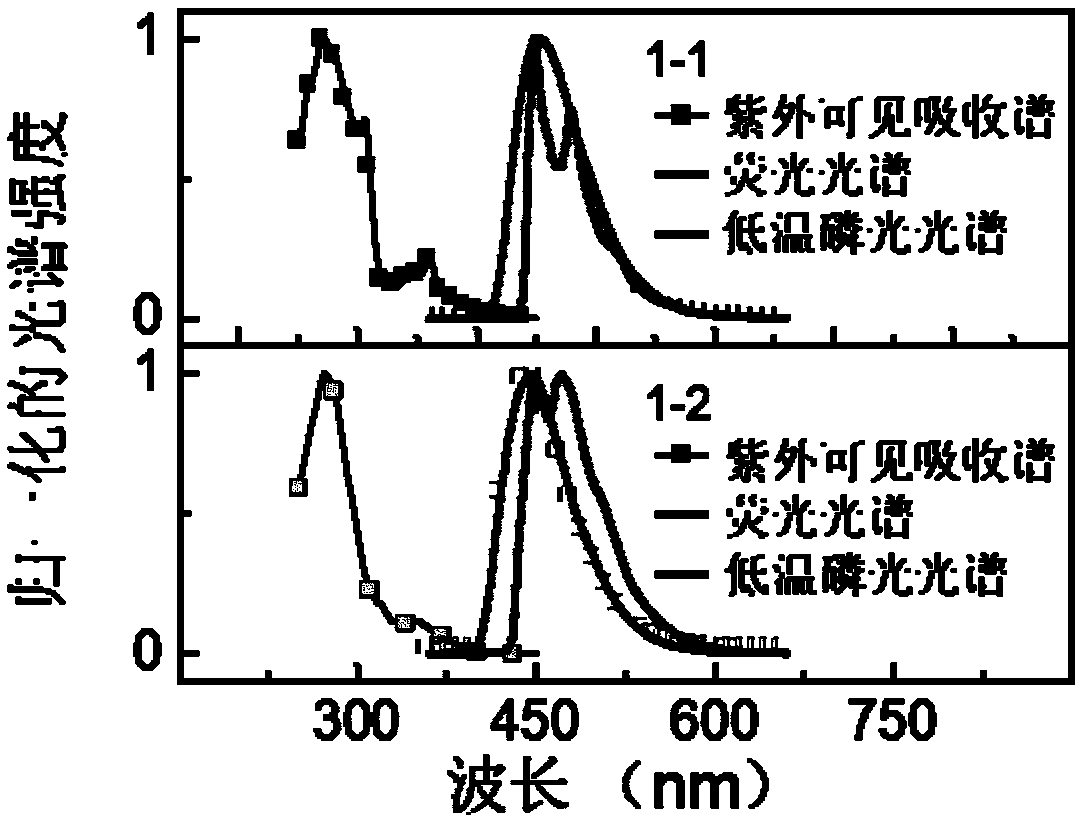
Thermal activation delayed fluorescent material and application of thermal activation delayed fluorescent material to organic light emitting diodes
A technology of thermally activated delayed and fluorescent materials, which is applied in the direction of luminescent materials, material analysis through optical means, and analysis of materials, etc., which can solve problems such as the efficiency and lifespan of blue light materials are not too mature, reducing plane conjugation, unfavorable white light lighting fields, etc. , to achieve the effect of excellent carrier transport ability, favorable hole injection and good carrier transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of intermediate 1 (2-(4-fluoro-3-methylphenyl)-4,6-diphenyl-1,3,5-triazine, TRZ-F)
[0038] Take 2.0 g of 2-chloro-4,6-diphenyl-1,3,5-triazine (7.49 mmol) into a dry 250 ml two-neck flask, then add 3.97 g of sodium carbonate and 100 mL of tetrahydrofuran and ethanol Mixed solution, 1.5 g (4-fluoro-3-methylphenyl)boronic acid (9.74 mmol), (volume ratio=4:1). Add 0.087 g of tetrakis(triphenylphosphine)palladium (0.075 mmol) after deoxygenation with nitrogen, stir and heat to reflux. After the reaction of 2-chloro-4,6-diphenyl-1,3,5-triazine was completely consumed, it was cooled to room temperature, and the obtained crude product was purified by column chromatography to obtain a white powder (2.2 g). The rate is 86.3%. MS (EI) m / z: 341.36[M + ].
Embodiment 2
[0039] Example 2: Synthesis of intermediate 2 (3-bromo-9H-fluorene):
[0040]
[0041] Add 0.62 g iodine (2.44 mmol) and 50 mL glacial acetic acid in sequence to a 250 mL round bottom flask (nitrogen), dissolve and add 50 wt% hypophosphorous acid aqueous solution (2.44 mmol) dropwise, then heat and stir (about 100 °C). After the reaction was completed, 0.52 g of 3-bromofluorenone (2.0 mmol) was added, and after the reaction of 3-bromofluorenone was complete, it was cooled. The reaction was also poured into pure water to precipitate a white solid, which was filtered with suction to obtain 0.45 g of a white solid, yield: 91.8%. MS (EI) m / z: 244.87[M + ].
Embodiment 3
[0042] Example 3: Synthesis of intermediate 3 (3-bromo-9, 9-dimethylfluorene):
[0043]
[0044] Add 1 g of 3-bromo-9H-fluorene (4.08 mmol), 2.3 g of sodium tert-butoxide (24.1 mmol) and 100 mL of anhydrous tetrahydrofuran to a 250 mL two-neck round-bottom flask (nitrogen), and stir to add 2.3 g Iodomethane (16.2 mmol) was reacted for 6h. After 3-bromo-9H-fluorene was completely consumed, 5 mL of water was added to quench the reaction, and 100 mL of dichloromethane was added. After two extractions, the organic phase was concentrated to obtain a crude product. The crude product was purified by column chromatography to obtain 0.95 g of a transparent oily product, yield: 85.3%. MS (EI) m / z: 272.54[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com