Lithium ferromanganese phosphate and preparation method thereof
A technology of lithium iron manganese phosphate and ferromanganese hydrogen phosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of complex recovery process, complex process, and high equipment requirements, and achieve high atomic economy and technology. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] A preparation method for lithium manganese iron phosphate, comprising the following steps:
[0039] (1) mixing elemental iron, manganese dioxide and phosphoric acid aqueous solution to obtain mixture A, and ball milling said mixture A to obtain ferromanganese phosphate;
[0040] (2) Contact the ferromanganese phosphate, lithium carbonate and glucose, sand-mill until the particle size D50 of the product is 0.2 μm-1 μm, dry, and calcinate to obtain lithium ferromanganese phosphate.
[0041] Wherein, the "ball milling" and "sand milling" can be carried out in a wide temperature range, the preferred temperature range is 10-60°C, and the milling time depends on whether the D50 particle size of the product has reached the required range, The preferred grinding time is 10h-50h.
[0042] The drying described in step (2) includes but is not limited to vacuum freeze drying, heating drying under nitrogen or inert gas protection, microwave drying, spray drying or flash drying, etc...
Embodiment 1
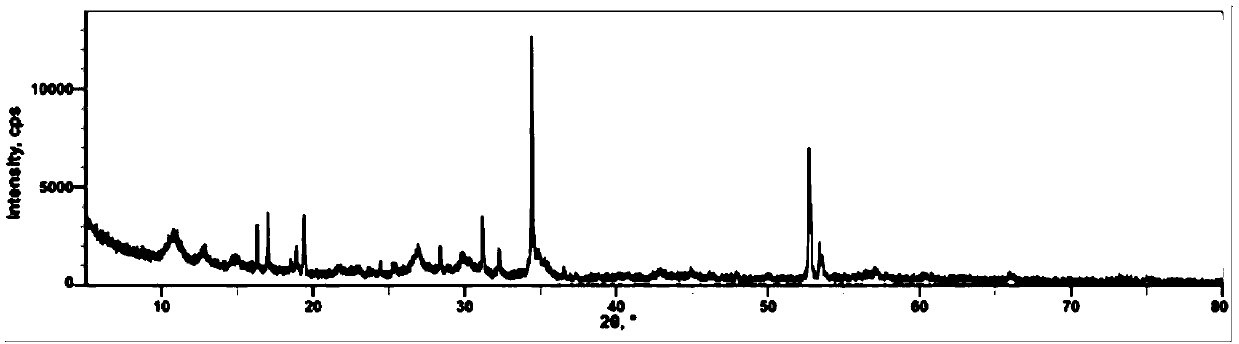
[0047] Step 1, take by weighing 0.3 mole of iron powder (Fe content > 99%), 0.2 mole of electrolytic manganese powder (Mn content > 99.8%), 0.5 mole of electrolytic manganese dioxide powder (MnO 2 Content > 99%) mixed with phosphoric acid aqueous solution (mass concentration 50%) containing 1.03 moles of phosphoric acid, placed in a ball mill for reaction at 25°C, after 15 hours, the particle size D50 of ferromanganese phosphate was measured to be 5 microns, and the ball milling was terminated . Carry out X-ray diffraction test (XRD) to ferromanganese phosphate powder, test pattern such as figure 1 shown.
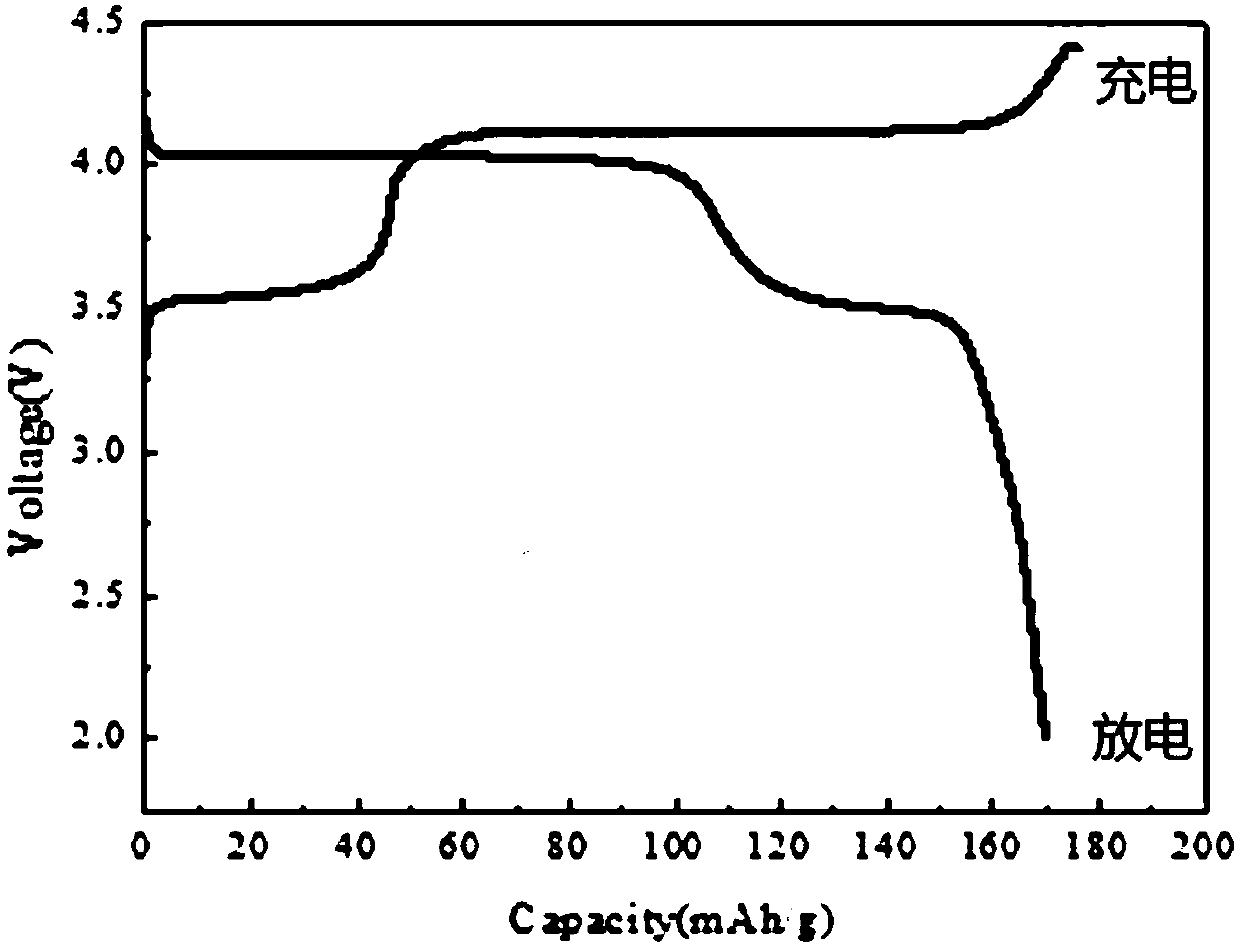
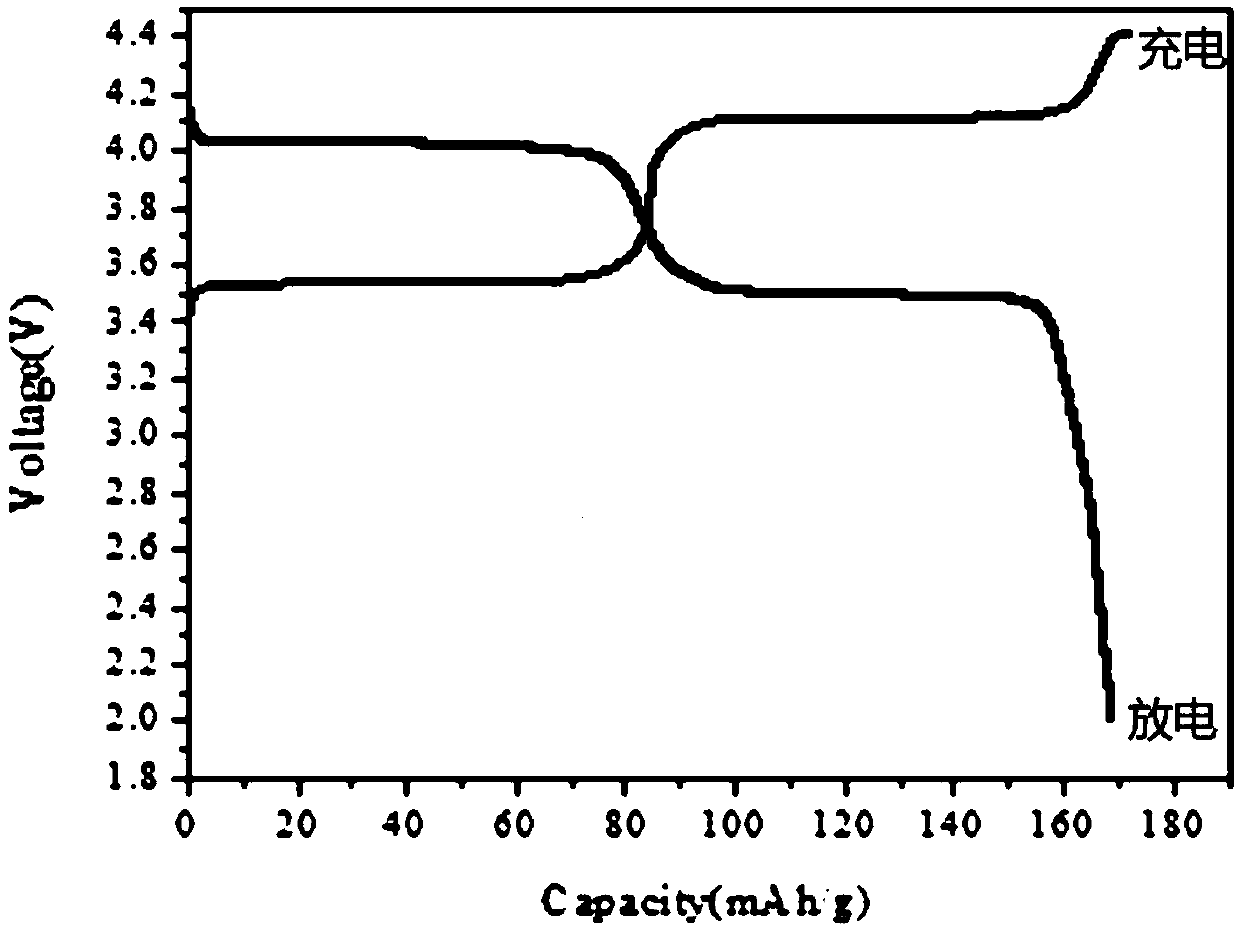
[0048]Step 2. Transfer the above-mentioned ferromanganese phosphate to a sand mill for reaction. Add 1.05 moles of lithium carbonate and 0.1 moles of glucose at 20°C. After 30 hours, it is detected that the particle size D50 of the material is 0.6 microns, and the sand milling is terminated. The slurry is spray-dried, the drying temperature is 250°C, and the drying time i...
Embodiment 2
[0050] Step 1, take by weighing 0.3 mole of iron powder (Fe content > 99%), 1.5 mole of electrolytic manganese dioxide powder (MnO 2 Content > 99%) mixed with phosphoric acid aqueous solution (mass concentration 10%) containing 3.06 moles of phosphoric acid, placed in a ball mill for reaction at 40°C, after 40 hours, the particle size D50 of ferromanganese phosphate was measured to be 2 microns, and the ball milling was terminated .
[0051] Step 2. Transfer the above-mentioned ferromanganese phosphate to a sand mill for reaction, add 3 moles of lithium carbonate and 1 mole of glucose at 35°C, and after 50 hours, it is detected that the particle size D50 of the material is 0.2 microns, and the sand milling is terminated. The slurry is spray-dried, the drying temperature is 200°C, and the drying time is 10 seconds. The dried powder is calcined at 700°C under the protection of nitrogen, and the calcining time is 8 hours. After cooling, take it out and put it in gloves. Make a b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com