Conjugated small molecule based on nona fused heterocycle, preparation method and applications thereof
A technology of small molecules and fused heterocyclic rings, which is applied in the field of organic solar material preparation to achieve strong absorption, good thermal stability, and high charge transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of 4-n-octyl-4H-dithieno[3,2-b:2',3'-d]pyrrole (1):
[0057]
[0058] In a 50mL two-neck flask, 3,3'-dibromo-2,2'-bithiophene (2.5g, 7.71mmol) and sodium tert-butoxide (1.85g, 19.29mmol) were dissolved in 15mL of toluene, and a nitrogen drum After soaking for 10 minutes, quickly add tris(dibenzylideneacetone)dipalladium (211.94mg, 0.231mmol) and 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (480.40mg, 0.771mmol) ), under the protection of nitrogen, slowly dropwise add n-octylamine (997.13 mg, 7.71 mmol) to the mixed solution, and heat to 110° C. to react overnight. Stop the reaction, cool to room temperature, pour the reaction mixture into deionized water, extract with ethyl acetate, wash twice with water, wash once with saturated brine, dry the organic phase with anhydrous magnesium sulfate, spin dry the organic solvent, and chromatographic column chromatography After separation, the eluent was petroleum ether / dichloromethane (volume ratio 9:1) to obtain pale yello...
Embodiment 2
[0072] Synthesis of compound INTC
[0073]
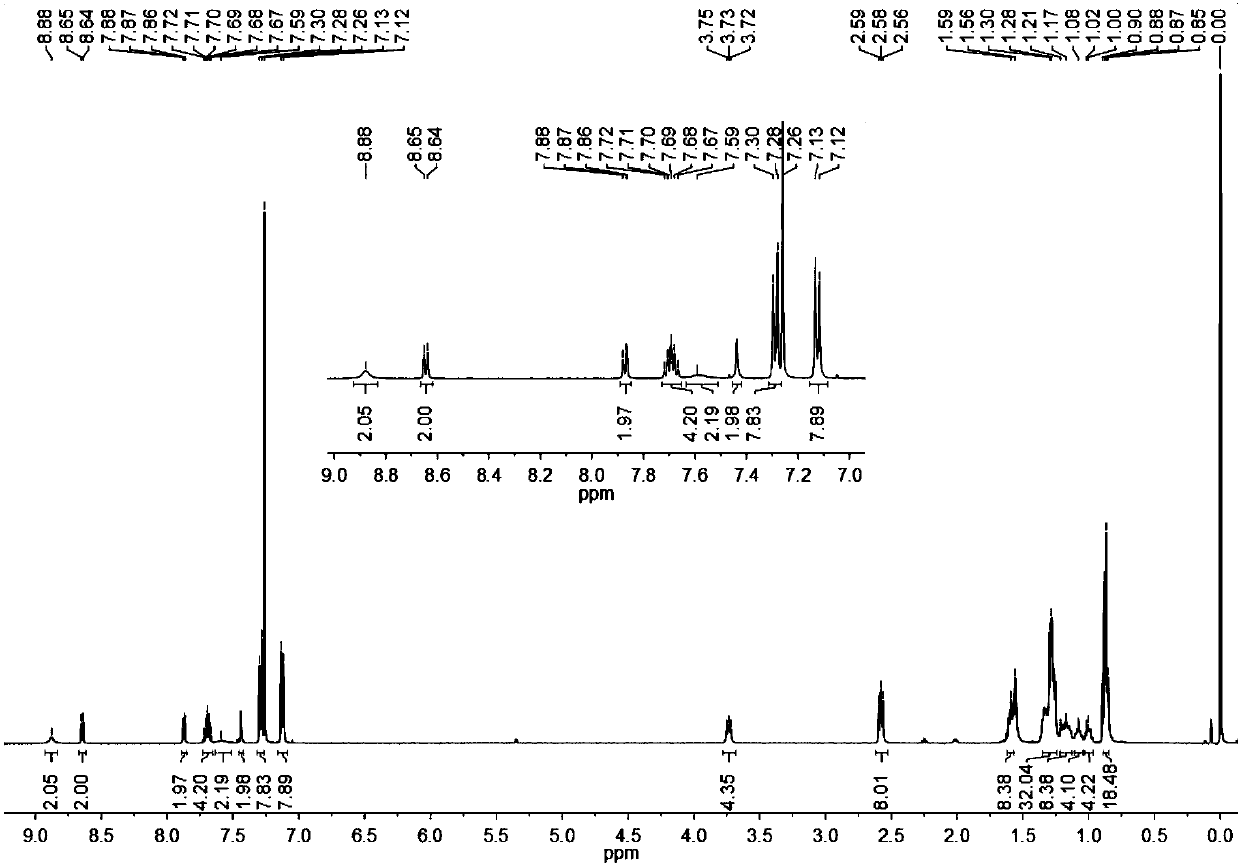
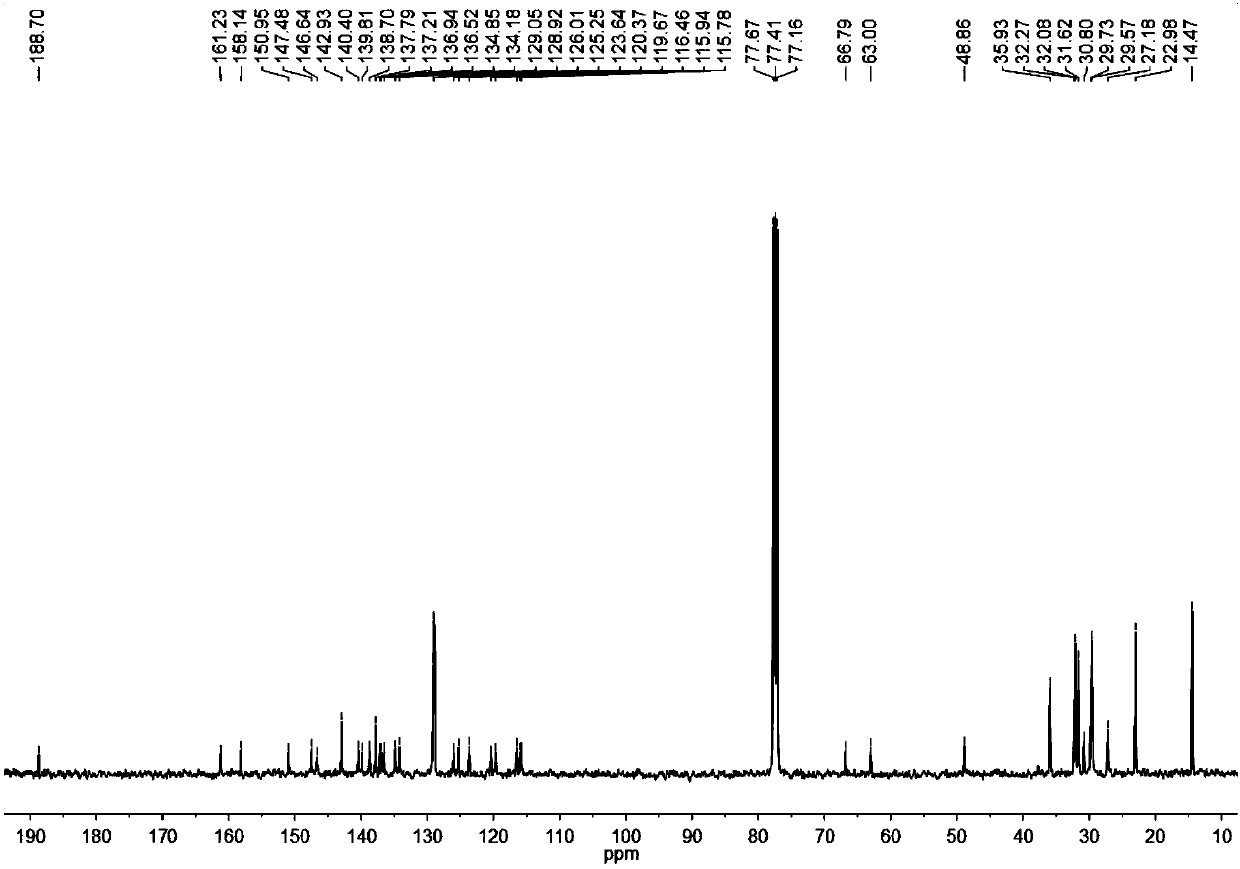
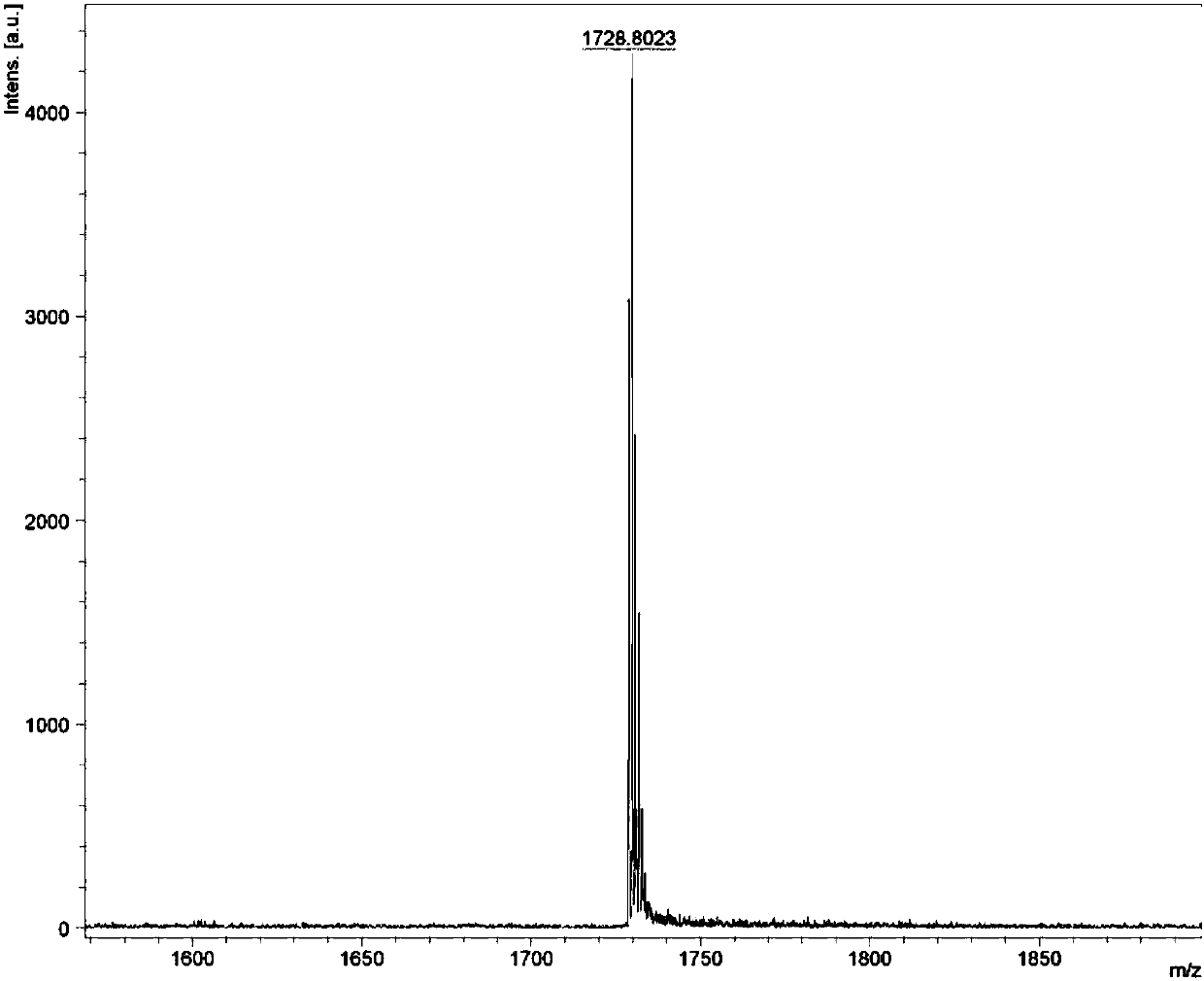
[0074] Under the protection of nitrogen, in a 25mL reaction tube, compound 5 (150.0mg, 108.85μmol), 3-(dicyanomethylene)indigo (211.37mg, 1.09mmol) and β-alanine (1.94mg, 21.77 μmol) is dissolved in a dry 1,2-dichloroethane / absolute ethanol (8ml / 4ml) mixed solvent. After the reaction was heated to reflux for 24 hours, it was cooled to room temperature. It was extracted three times with chloroform, the organic phase was washed twice with water, and dried with anhydrous magnesium sulfate. The solvent was removed, and the residue was separated by column chromatography. The eluent was petroleum ether / dichloromethane (volume ratio 2:1) to obtain a black-green solid INTC (128 mg, 68%). The hydrogen nuclear magnetic spectrum, carbon spectrum and high resolution mass spectrum of this compound are attached respectively. figure 1 , Attached figure 2 , Attached image 3 . 1 H NMR(500MHz, CDCl 3 )δ (ppm) = 8.88 (s, 2H), 8.64 (d, J = 6.7 Hz, 2H)...
Embodiment 3
[0076] Synthesis of compound INTC
[0077]
[0078] Add compound 5 (150.0 mg, 108.85 μmol), 3-(dicyanomethylene) indigo (211.37 mg, 1.09 mmol), 30 mL of chloroform into a 50 mL two-necked flask, and blow nitrogen for 30 minutes to remove the air in the flask After adding 1 mL of pyridine, stirring and reacting at 65°C for 24 hours, and then cooling to room temperature, the resulting reaction solution was poured into 200 mL of methanol, and the precipitate obtained by filtration was dried and separated by silica gel column chromatography. The eluent was petroleum ether / Dichloromethane (volume ratio 2:1), the product is a black-green solid INTC (120 mg, 64%). The hydrogen nuclear magnetic spectrum, carbon spectrum and mass spectrum are the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com