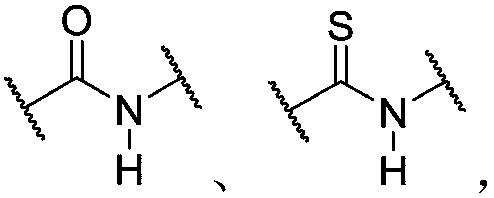
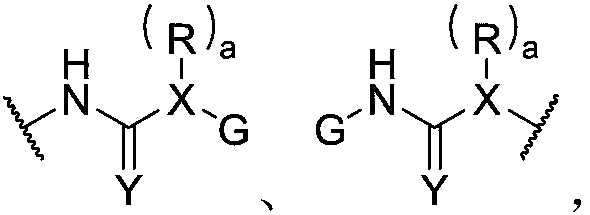Hybrid dynamic polymer composition and applications thereof
A polymer and composition technology, applied in the field of intelligent polymers, can solve the problems of lack of dynamics in chemical crosslinking, lack of dimensional stability, and inability to change crosslinking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0204] A preferred preparation method of a hybrid dynamic polymer composition ionic liquid gel of the present invention includes but not limited to the following steps: each raw material and other raw materials for preparing the hybrid dynamic polymer composition components A and B and other raw materials are added to the ion In the liquid, the sum of the mass fractions of components A and B of the prepared hybrid dynamic polymer composition is 0.5 to 70%, and polymerization, coupling, crosslinking or other types of chemical reactions are carried out by the appropriate means, and the reaction After the end, a hybrid dynamic polymer composition ionic liquid gel is made. A preferred preparation method of another hybrid dynamic polymer composition ionic liquid gel of the present invention includes but not limited to the following steps: the hybrid dynamic polymer composition components A, B and other raw materials are swollen in In the ionic liquid or the solvent containing the i...
Embodiment 1
[0264] Preparation of a polynorbornene-based hybrid dynamic polymer composition plasticizer-swellable gel in which supramolecular interactions are based on pendant cyanuric acid groups.
[0265] Cyanuric acid and 6-chloro-1-hexene were kept at a molar ratio of 4:1, dissolved in anhydrous dimethyl sulfoxide, and stirred and reacted at 80°C for 15 hours under the catalysis of potassium carbonate to obtain a hydrogen-bonding group group of olefin monomers 1a. Add compound 1a to toluene, cool the reaction vessel to 5°C, and add cyclopentadiene dropwise with stirring at low temperature, keeping the molar ratio of compound 1a to cyclopentadiene at 10:13. After the dropwise addition, the temperature was raised to reflux temperature and the reaction was continued with stirring to obtain a norbornene derivative 1b containing a hydrogen bond group.
[0266]
[0267] 30 molar equivalents of norbornene derivative 1b containing hydrogen bonding groups, 65 molar equivalents of norbornen...
Embodiment 2
[0271] A polyacetylene-based hybrid dynamic polymer composition hydrogel with supramolecular interactions based on pendant imidazolinone groups, pendant amide groups, and backbone amide groups was prepared.
[0272] Under nitrogen protection, a certain amount of p-acetylene benzoic acid was dissolved in sodium hydroxide aqueous solution (sodium hydroxide concentration was 10M), keeping the molar ratio of monomer to sodium hydroxide at 2:3. Add catalyst [Rh(cod) at 30°C 2 ] BF 4 aqueous solution, adjust the monomer and catalyst concentrations to 0.5M and 0.0025M, respectively. After 3 hours, the resulting polymer was precipitated in ethanol, dried and dissolved in water again, acidified with hydrochloric acid and centrifuged to obtain poly[(4-carboxyphenyl)acetylene].
[0273] Under anhydrous and oxygen-free conditions, a certain amount of the obtained polymer was dissolved in DMF, and at -60°C, 1-(2-aminoethyl)-2-imidazolidinone with 0.4 molar equivalents of carboxyl groups ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com