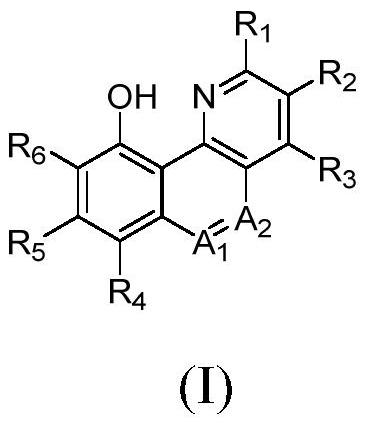
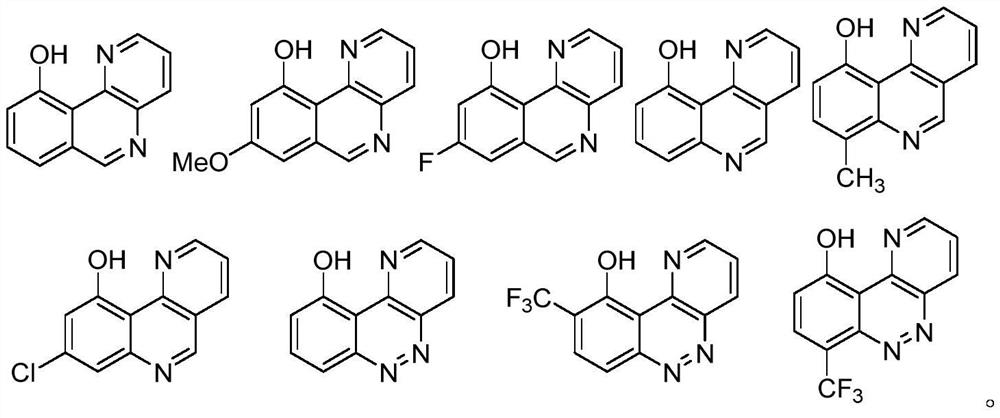
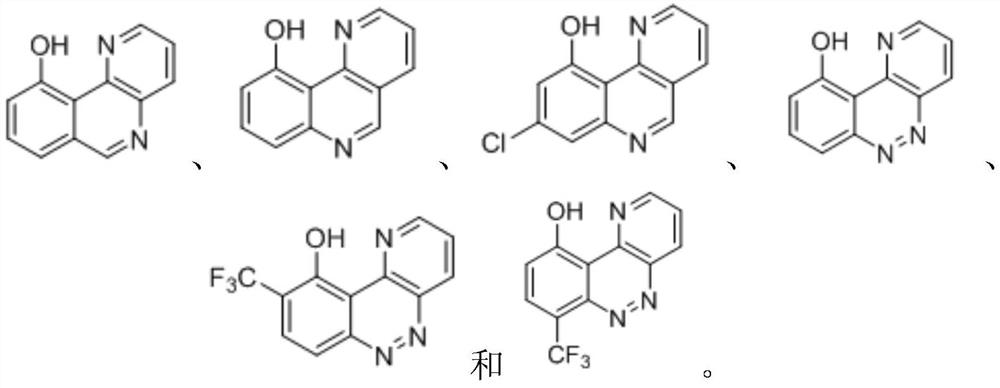
Benzoheterocyclic compounds and their applications
A compound and extraction agent technology, applied to benzoheterocyclic compounds and their application in the extraction and separation of lithium isotopes, can solve problems such as inability to meet product requirements, large amounts of mercury, pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The preparation of formula (I) compound
[0069] The compounds of the present invention can be prepared by standard methods known in the art or by known methods analogous thereto. General methods for preparing the compounds of the invention are set forth below. The starting materials and reagents described in the following experimental reaction schemes are generally commercially available.
[0070] (1) When the compound: A 1 is a CH group and A 2 When it is an N atom, its general synthesis method is as follows:
[0071]
[0072] In the above reaction scheme, the first step adopts the conventional amine group protection method to carry out group protection. The second step reaction is a Pd-catalyzed coupling reaction (reference: US patent application US20080214818). The third step reaction is the hydroxylation reaction catalyzed by Pd, and the D-type compound is finally synthesized.
[0073] Among them, another preparation route of compound C can also refer to t...
Embodiment 1
[0118] The synthetic method of compound D1:
[0119]
[0120] Step 1: Dissolve 650 mg of the commercially available compound 2-chloro-3-aminopyridine (A1, 5 mmol, 1.0 equiv.) in 10 mL of triethylamine, add 1 mL of acetyl chloride (15 mmol, 3.0 equiv.) under nitrogen protection, and After stirring for 3 hours, 1 mL of acetyl chloride (15 mmol, 3.0 equiv.) was added, and the reaction was continued at room temperature for 15 hours. TLC detected that the raw materials were almost completely reacted. Add 50 mL of water and 50 mL of ethyl acetate for liquid separation and extraction. The aqueous phase after liquid separation is extracted twice with 50 mL of ethyl acetate, and the combined organic phase is washed twice with 50 mL of water. After drying, the solvent is evaporated to obtain 701 mg of yellow oil B1 in total. , and the yield was 82%, which can be directly used in the next reaction.
[0121] The second step: 700mg (3.89mmol, 1.0equiv) of the above-mentioned oil, 750mg...
Embodiment 2
[0124] The synthetic method of compound G1:
[0125]
[0126] Step 1: Add 8.5g of compound E1, 15mL of glycerol, 15mL of nitrobenzene, 15mL of concentrated sulfuric acid, and 10ml of ice water into the reaction flask, and heat to 160°C for 24 hours. After cooling to room temperature, add 4M NaOH to neutralize, add dichloromethane and water to extract the viscous liquid four times, combine the organic phases and wash with water twice, evaporate the solvent after drying, and separate compound F1 by column chromatography, solid 3.82g , yield 35%.
[0127] The second step: add 0.180g (1.0mmol) of compound F1, 0.676g (2.0mmol) of iodobenzene acetate, 0.012g of palladium acetate (0.042mmol) in the sealed tube, and add 5mL of acetonitrile and 0.4mL of acetic anhydride, mix Heated to 165°C and stirred for 18h. Acetonitrile was evaporated, 0.2g sodium hydroxide and 10mL methanol were added, hydrolyzed, neutralized, extracted with dichloromethane to obtain an organic layer, washed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com