Drug balloon and preparation method thereof
A drug and balloon technology, applied in the field of medical devices, can solve the problems of increasing the risk of vascular tissue inflammation and thrombus occlusion, increasing thrombus occlusion, apoptosis of vascular tissue cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the above-mentioned pharmaceutical balloon comprises the following steps:
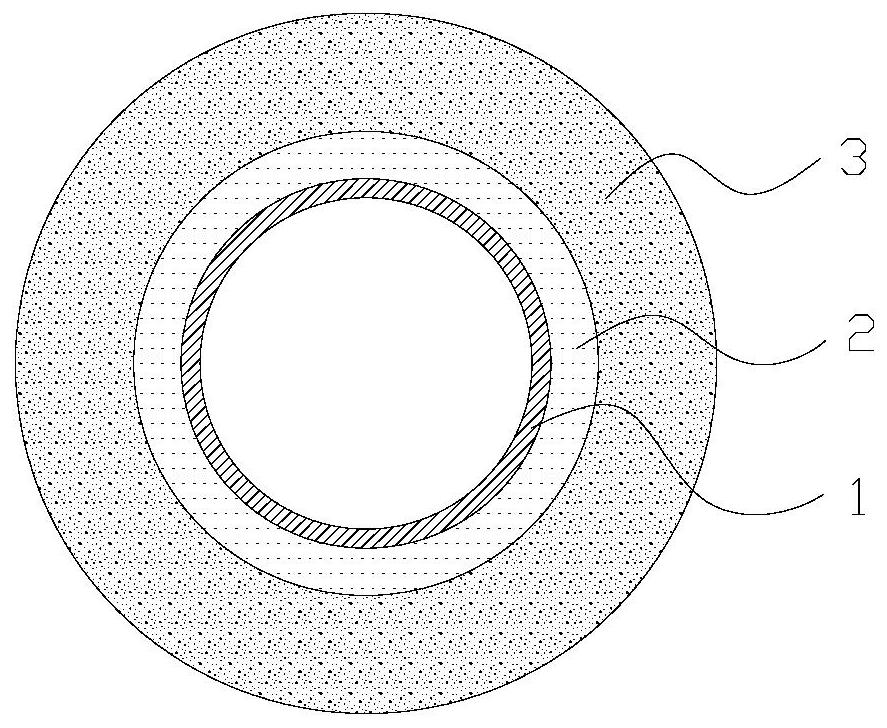
[0027] Step S210 , pretreating the surface of the balloon 1 to make the surface of the balloon 1 hydrophilic and rough.
[0028] In one embodiment, the balloon is a nylon balloon.
[0029] In one embodiment, the pretreatment includes at least one of alcoholization treatment, plasma treatment and etching groove treatment.
[0030] Further, the alcoholization treatment is as follows: at 10°C to 70°C, the balloon 1 is immersed in an aqueous solution of ethanol with a volume concentration of 50% to 99.5% for 5 minutes to 120 minutes, taken out and dried.
[0031]The process parameters of the plasma treatment are as follows: the gas used is at least one of nitrogen, oxygen and argon, the output power is 50W to 2000W, the frequency is 10MHz to 100MHz, the treatment time is 5 seconds to 30 minutes, and the air pressure is 1Pa to 100Pa.
[0032] It can be understood that step...
Embodiment 1
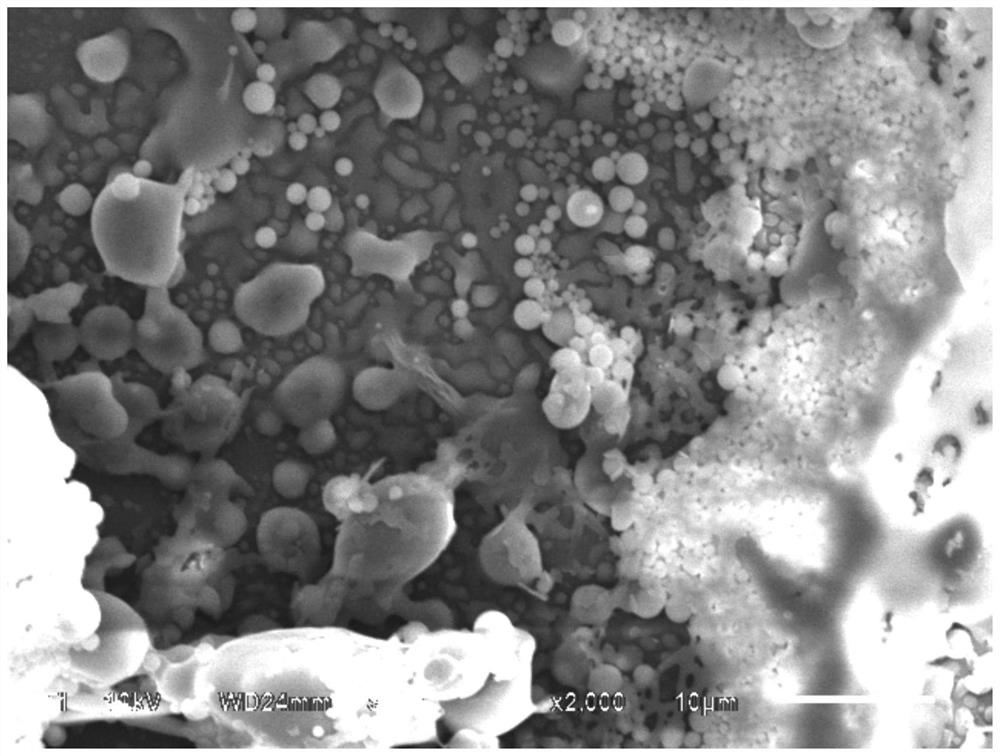
[0091] A peripheral PTA balloon catheter with a balloon size of 4.0 × 40 was selected. In a 10,000-level clean environment, a plasma machine was used to pretreat the surface of the PTA balloon catheter (diameter 4mm, length 40mm, nylon balloon), and plasma pretreatment gas It is a mixture of argon and oxygen, the volume ratio V 氩气 : V 氧气 =1:1, the plasma processing power is 500W, the frequency is 30MHz, the processing time is 10min, and the air pressure is 50Pa.
[0092] The commercially available silane coupling agent KH-602 is used to prepare the treatment solution, and the solvent is a mixed solvent of methanol, ethanol and water for injection. The volume percentage is 17%, the volume percentage of water for injection is 10%, and the volume percentage of glacial acetic acid used as a silane coupling agent hydrolysis catalyst is 1%. Soak the balloon part of the plasma-treated balloon catheter into the treatment solution, slowly take out the balloon from the solution after ...
Embodiment 2
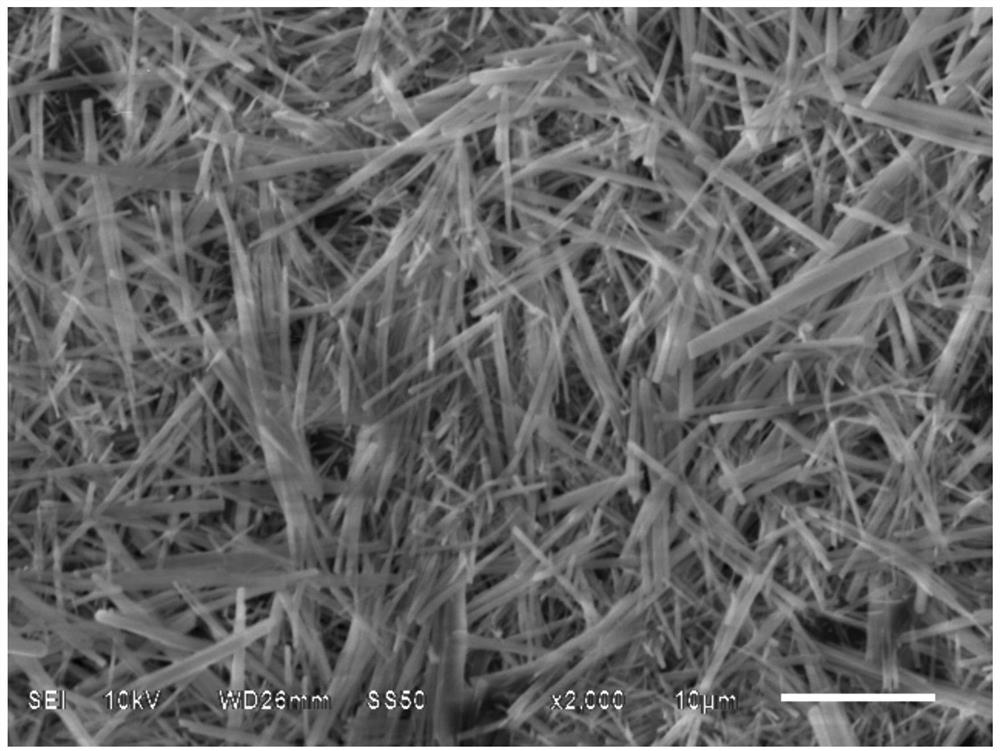
[0096] In this example, a peripheral PTA balloon catheter with a balloon size of 4.0×40 is selected. In a 10,000-level clean environment, a plasma machine is used to perform surface pretreatment on the PTA balloon catheter (diameter 4mm, length 40mm, nylon balloon). Plasma The pretreatment gas is a mixture of nitrogen and oxygen, with a volume ratio of V 氮气 : V 氧气 =1:2, the plasma processing power is 50W, the frequency is 100MHz, the processing time is 30min, and the air pressure is 50Pa.
[0097] The commercially available silane coupling agent KH-792 is used to prepare the treatment solution. The solvent is a mixed solvent of ethanol, isopropanol and water for injection. The volume percentage of KH-792 in the treatment solution is 1.5%, and the volume percentage of ethanol is 55%. The volume percentage of isopropanol is 33%, the volume percentage of water for injection is 10%, and the volume percentage of glacial acetic acid as a silane coupling agent hydrolysis catalyst is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com