Dendritic molecular organic fluorescent material, application thereof, fluorescent film and preparation method of fluorescent film
A fluorescent material and dendritic technology, applied in the field of fluorescent sensing, can solve the problems of poor stability, high toxicity of conjugated polymers, and low sensitivity, and achieve high efficiency, good photochemical stability, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The present invention has no special requirements for the synthesis method of the dendritic molecular organic fluorescent material described in the above scheme, and it is only necessary to use a method well known to those skilled in the art to synthesize the dendritic molecular organic fluorescent material that can obtain the above-mentioned structure. In the present invention , the preferred synthetic method comprises the following steps:
[0064] When n=1 in formula I, the synthetic method of described dendrimer organic fluorescent material preferably comprises the following steps:
[0065] (a) Under the action of an alkaline substance, make C-Br and bis-boronate carry out boronation reaction in an organic solvent to obtain a compound having a structure shown in Formula 1;
[0066]
[0067] (b) under nitrogen protection conditions, Br-B-I2, compound shown in formula 1, basic substance, palladium catalyst and organic solvent are mixed to carry out Suzuki reaction, ...
Embodiment 1
[0120]
[0121] The synthesis (denoted as G2) comprises the following steps:
[0122] (1) Synthesis of compound 9-(4-bromophenyl)-9H-carbazole
[0123]
[0124] Under nitrogen protection, carbazole (5g, 30mmol), p-fluorobromobenzene (10.47g, 60mmol), and cesium carbonate (21.5g, 62mmol) were added to a 250mL single-necked flask and mixed in DMSO (100mL) , reacted at 160°C for 48 hours, cooled to room temperature, extracted with dichloro / water solution, passed through the column with dichloromethane / petroleum ether, and dried in vacuo to obtain 7.7 g of white crystals with a yield of 80%.
[0125] 1 H NMR (500MHz, CDCl3) δ8.13 (d, J = 7.7Hz, 1H), 7.72 (d, J = 8.5Hz, 1H), 7.48–7.35 (m, 3H), 7.32–7.26 (m, 1H) .Mass spectrum molecular ion peak: 319.2, actual molecular weight: 322.2
[0126] (2) Synthesis of 9-(4-(4,4,5,5-tetramethyl-1,3,2-dioxoboronyl)phenyl)-9H-carbazole (referred to as G1-Bn2)
[0127]
[0128] Under nitrogen protection, p-bromocarbazole (4g, 12.4mm...
Embodiment 2
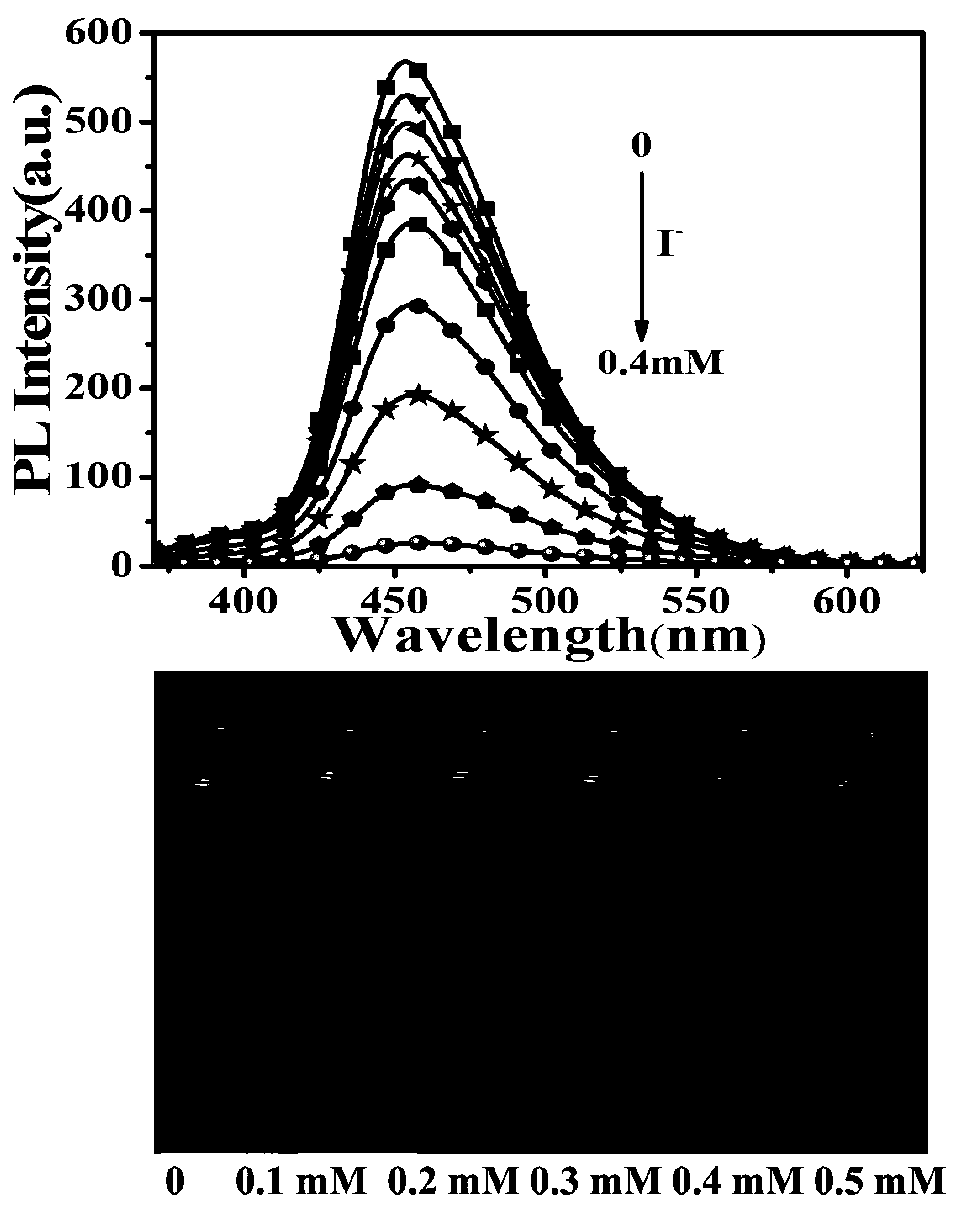
[0147] (1) 1.1 mg of the dendrimer G2 prepared in Example 1 was dissolved in 0.35 mL of tetrahydrofuran to form 10 -3 mol / L dendrimer base solution, and then take 3 μL and place it in a quartz cuvette containing 3mL of the mixed solution (the volume ratio of tetrahydrofuran to water is 4:1), thereby obtaining 10 -6 mol / L dendrimer solution.
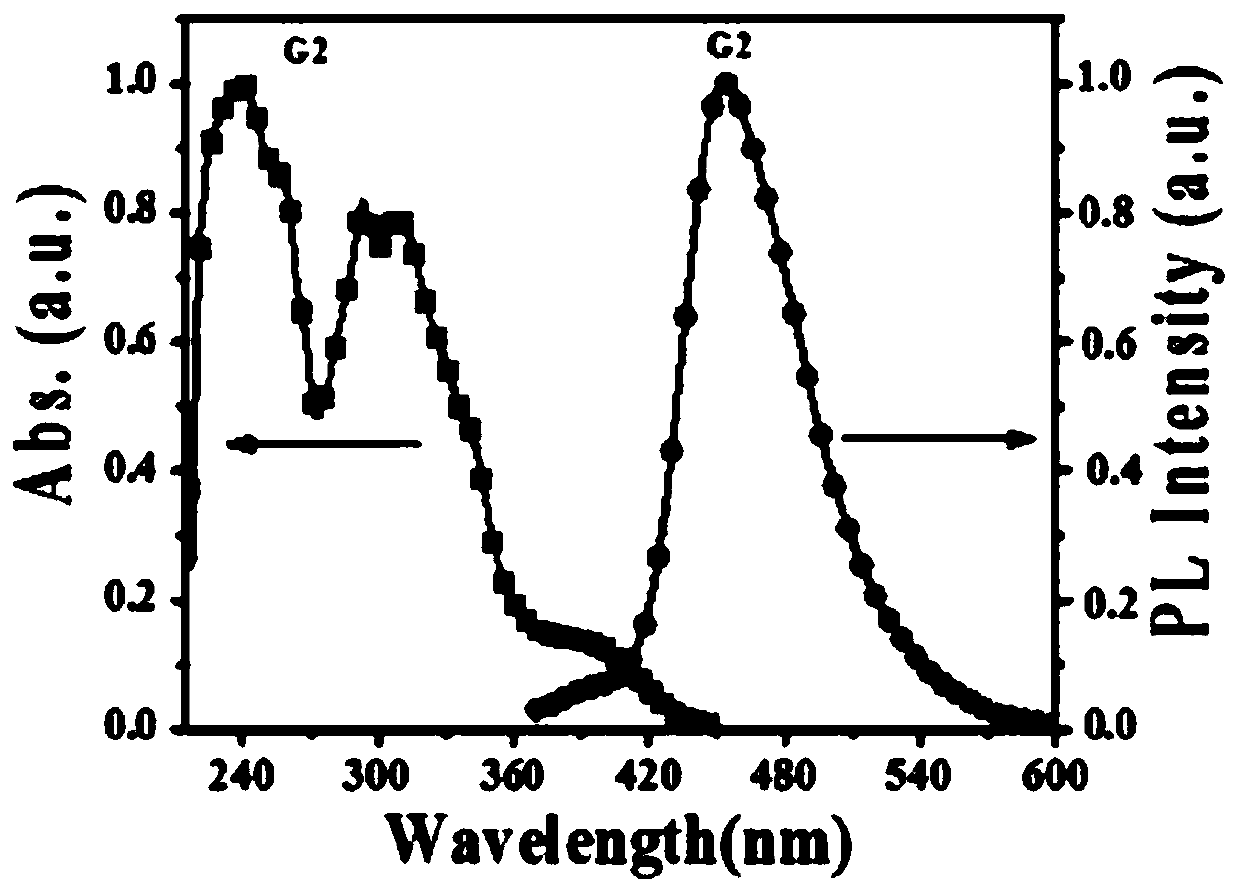
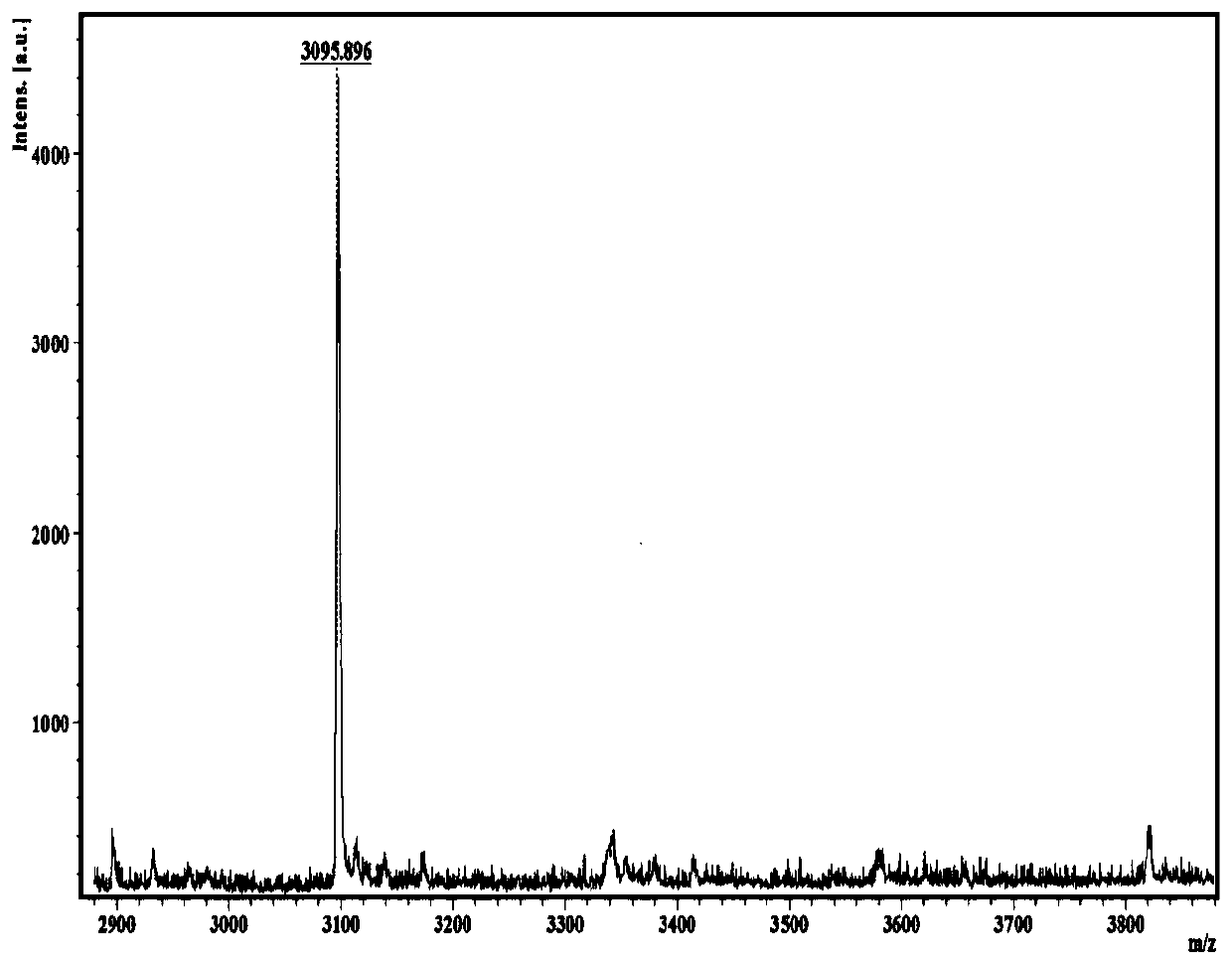
[0148] Record the ultraviolet absorption spectrum and the fluorescence emission spectrum of this dendrimer solution with a fluorescence spectrometer, and the obtained results are as follows: figure 1 As shown, the G2 mass spectrum was recorded with a matrix-assisted laser desorption time-of-flight mass spectrometer, and the obtained results are as follows figure 2 shown. according to figure 1 It can be seen that G2 has good optical properties, the band of about 394nm in the absorption spectrum comes from the pyrene nucleus, and the band of 301-350nm belongs to the absorption of carbazoles. Under excitation at 360nm, the PL spectrum G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com