OMS-2 type composite material with mixed crystal phase as well as preparation method and application of OMS-2 type composite material
A technology of OMS-2 and composite materials, applied in the field of nanomaterials and catalytic materials, can solve the problems of insufficient surface chemistry, singleness, and limited catalytic activity of materials, achieve excellent catalytic oxidation activity, simple operation method, and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] One aspect of the embodiments of the present invention provides a method for preparing an OMS-2 type composite material with a mixed crystalline phase, which includes:
[0025] Reacting the uniformly mixed reaction system containing potassium permanganate, divalent manganese salt, additives and nitric acid at 25-100°C for 5-24 hours to obtain an OMS-2 type composite material with mixed crystalline phases;
[0026] Wherein, the additive includes any one or a combination of two or more of tungstic acid, sodium phosphotungstate, sodium tungstate, sodium metatungstate, sodium phosphate, potassium phosphate, potassium dihydrogen phosphate and disodium hydrogen phosphate.
[0027] In some typical embodiments, the preparation method specifically includes: uniformly mixing an aqueous potassium permanganate solution with an aqueous solution containing divalent manganese salts, additives and nitric acid at 25-50°C to form the uniform mixing reaction system.
[0028] In some embo...
Embodiment 1
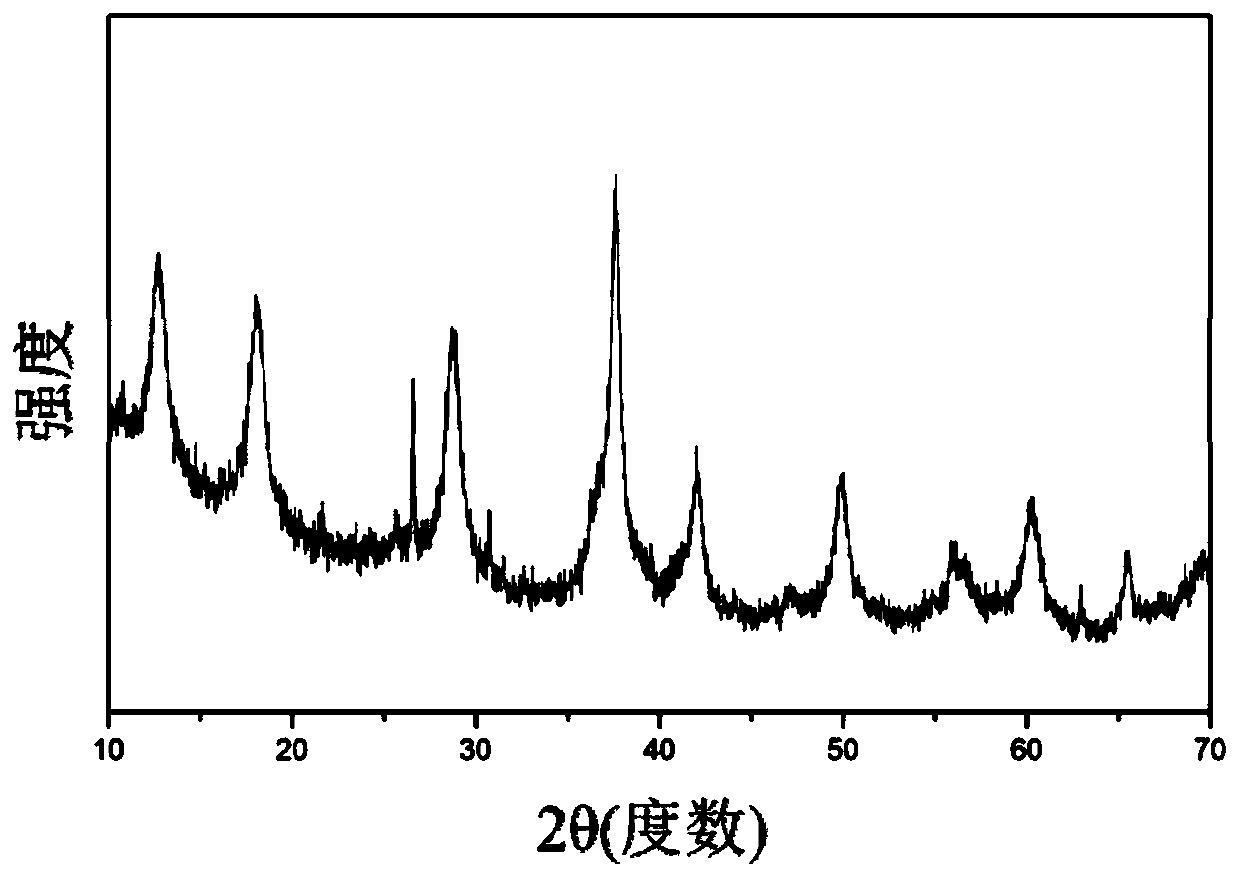
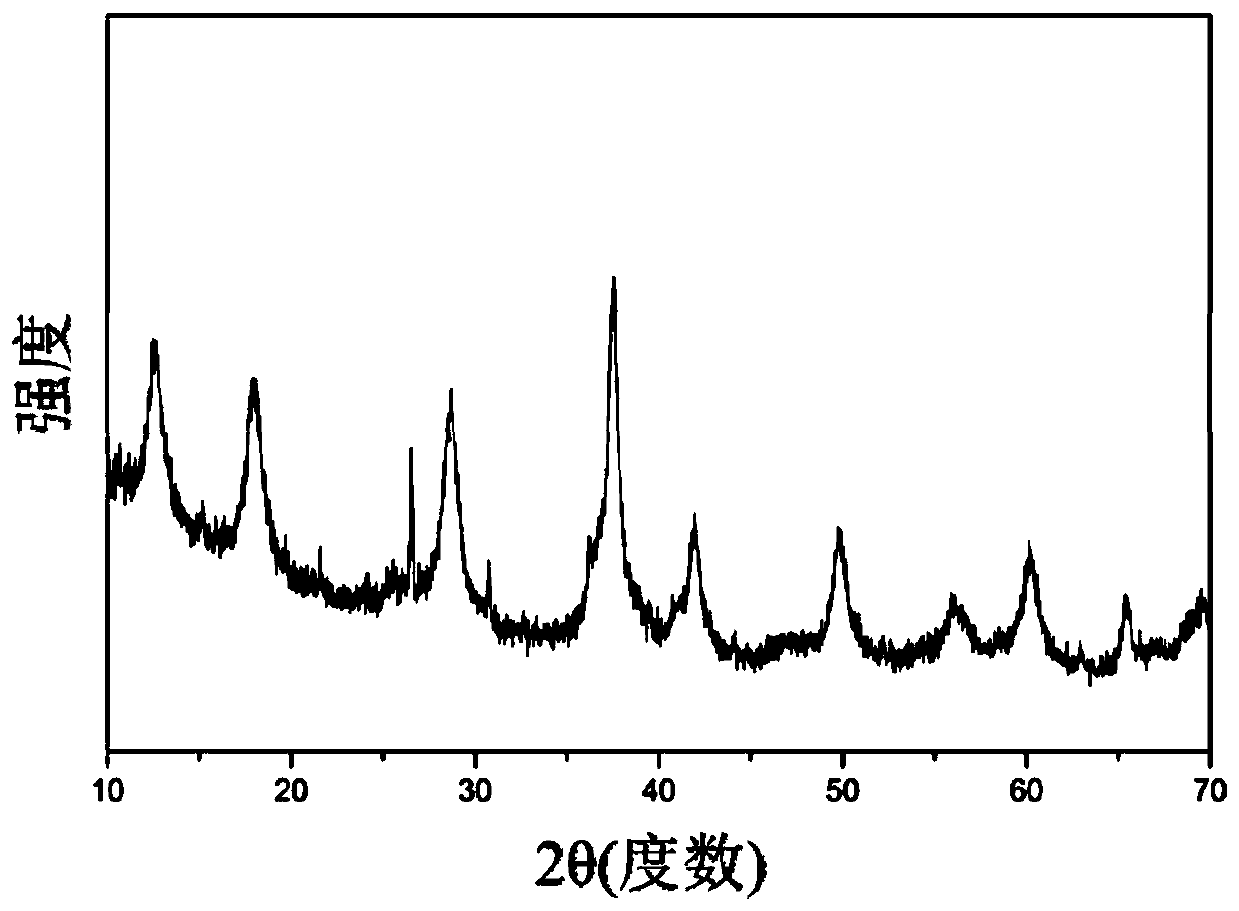
[0045] (1) At room temperature, dissolve 0.5mol manganese sulfate, 0.3mmol tungstic acid and 20mL nitric acid in 400mL distilled water, add 1L distilled aqueous solution containing 0.3mol potassium permanganate dropwise into the above mixed solution, stir and mix evenly, and then Stir and react at 80°C for 20 hours, filter, wash with distilled water, and dry at 120°C for 5 hours to obtain an OMS-2 composite material. Material phase identification: the XRD spectrum of the OMS-2 type composite material obtained in this embodiment is as follows figure 1 shown.
[0046] (2) Catalytic application
[0047] The selective oxidation of p-chlorobenzyl alcohol to p-chlorobenzaldehyde was used to consider the catalytic oxidation activity of the OMS-2 type composite material prepared in this example. Add 2mmol of p-chlorobenzyl alcohol, 50mg of OMS-2 type composite material as a catalyst, 3mL of dimethyl carbonate in the reaction bottle, stir and react at 90°C for 18 hours in an oxygen a...
Embodiment 2
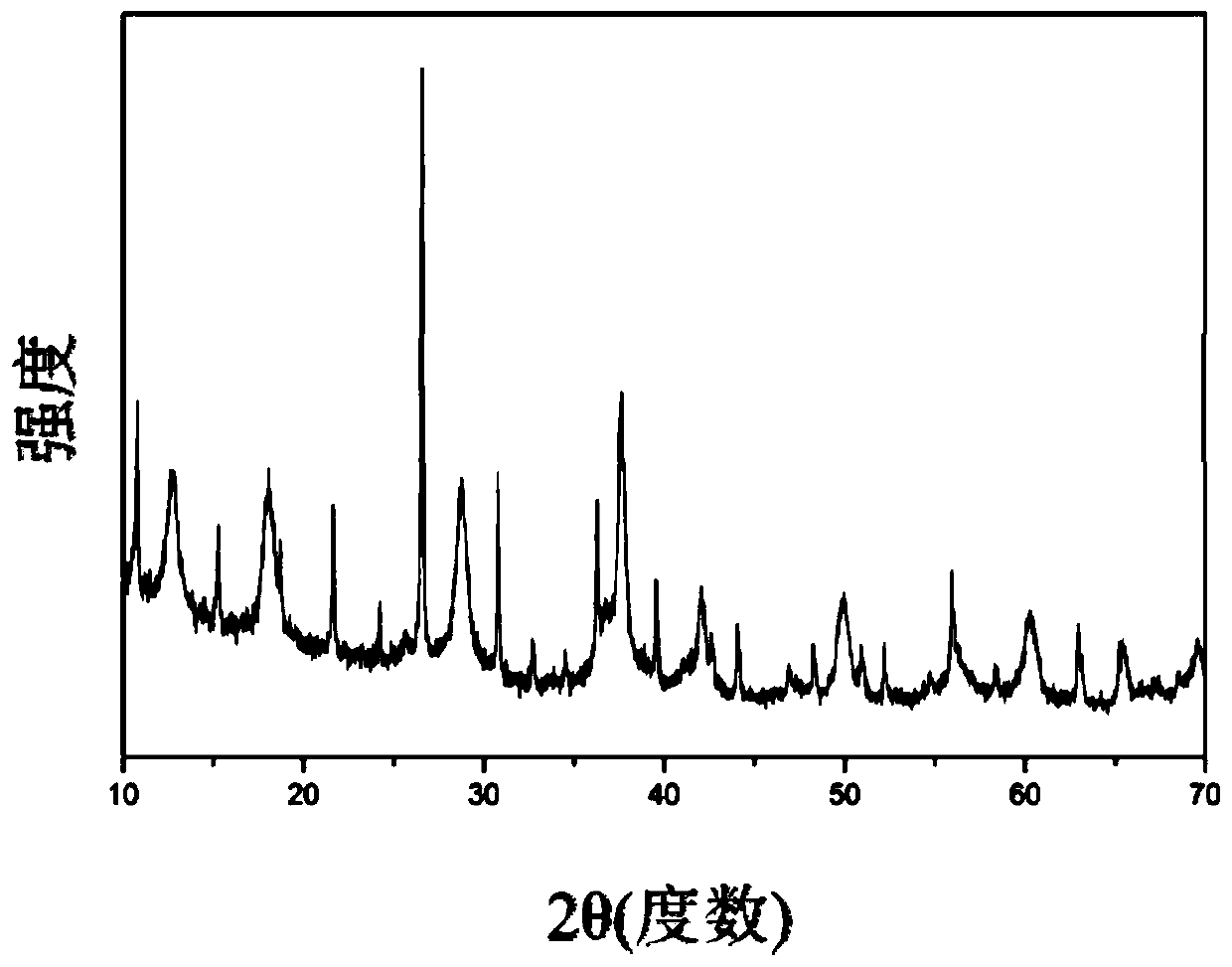
[0049] (1) At room temperature, dissolve 0.6mol manganese nitrate, 3mmol sodium tungstate and 30mL nitric acid in 300mL distilled water, add 1L distilled aqueous solution containing 0.4mol potassium permanganate dropwise into the above mixed solution, stir and mix evenly, and then Stir and react at 100°C for 24 hours, filter, wash with distilled water, and dry at 100°C for 12 hours to obtain an OMS-2 composite material. The XRD spectrum pattern of the OMS-2 type composite material that present embodiment obtains is as figure 2 shown.
[0050] (2) Catalytic application
[0051] The selective oxidation of benzyl alcohol to benzaldehyde was used to evaluate the catalytic oxidation activity of the OMS-2 type composite material prepared in this example. Add 0.02mol benzyl alcohol, 0.6g OMS-2 type composite material as catalyst, 50mL dimethyl carbonate in the reaction flask, stir and react at 90°C for 20 hours in an oxygen atmosphere, filter and remove the solid catalyst after th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com