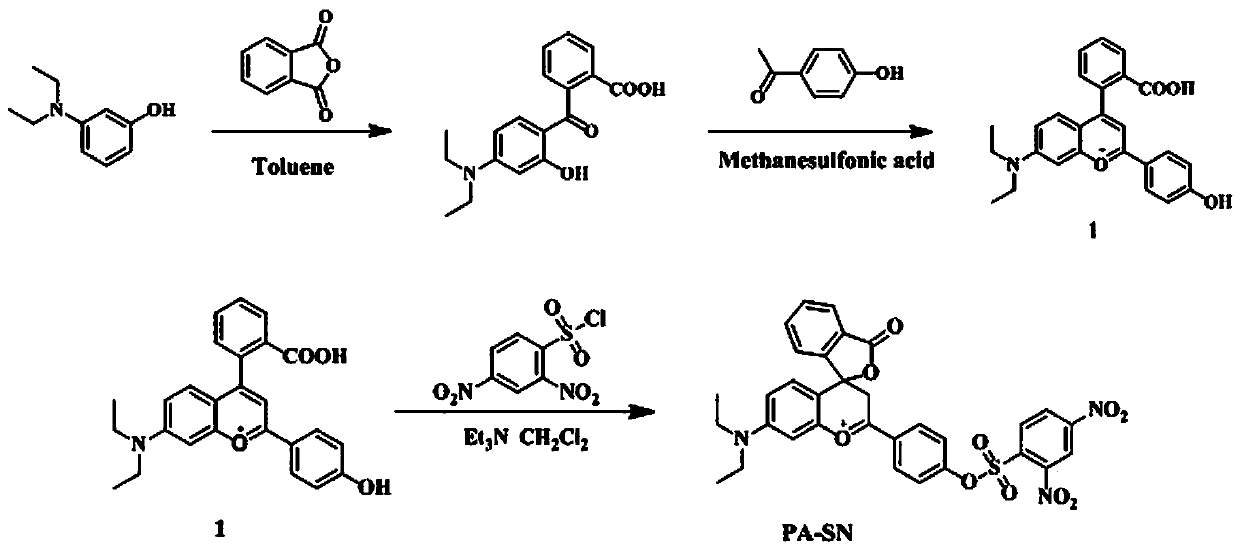
Fluorescent probe for detecting biological mercaptan in water-soluble environment and preparation method and application thereof
A technology of fluorescent probes and biothiols, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex synthesis process, low sensitivity, long response time, etc., and achieve high detection sensitivity and selectivity High and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound 1:
[0029] N-diethylaminophenol (1.8 g, 10.9 mmol) and phthalic anhydride (1.8 g, 12.2 mmol) were reacted in 200 mL benzene at 130 ° C for 12 hours to obtain a white solid, and then the white solid (0.626 g , 2 mmol) into 10 mL of methanesulfonic acid containing p-hydroxyacetophenone (0.272 g, 2 mmol), react at 90 °C for 8 hours, add to 200 mL of ice water after cooling, add 10 mL of perchloric acid, and dichloro After extraction with methane (50 mL × 3), drying and rotary evaporation to dry the solvent, compound 1 was obtained by column chromatography with a mixed solvent of dichloromethane and glacial acetic acid, yield: 33%. Synthetic route such as figure 1 shown.
Embodiment 2
[0030] Embodiment 2: Preparation of fluorescent probes for detecting biothiols in water-soluble environments according to the present invention:
[0031] Compound 1 (600 mg, 1.45 mmol), 2,4-dinitrobenzenesulfonyl chloride (1.542 g, 5.8 mmol), triethylamine (804 μL, 5.8 mmol) were dissolved in 30 mL redistilled dichloromethane, ice After bathing for 1 hour, return to room temperature and stir for 16 hours. Diluted with 30 mL of dichloromethane, washed with water (50 mL × 3), dried and evaporated to dryness, and separated by column chromatography with a mixed solvent of dichloromethane and methanol to obtain the target compound PA-SN, yield: 50%. 1 H NMR (600 MHz, CDCl 3 ) δ( ppm ) :8.68 (d, J = 1.8 Hz, 1H), 8.52 (dd, J = 8.6, 1.9 Hz, 1H), 8.41 (s, 2H), 8.21(d, J = 8.6 Hz, 1H), 7.99 (d, J = 7.7 Hz, 1H), 7.84 (d, J = 8.7 Hz, 2H), 7.67(t, J = 7.5 Hz, 1H), 7.60 (d, J = 7.5 Hz, 1H), 6.63 (d, J = 8.9 Hz, 1H), 6.47(dd, J = 18.9, 10.0 Hz, 2H), 5.65 (s, 1H), 5.32 (s, ...
Embodiment 3
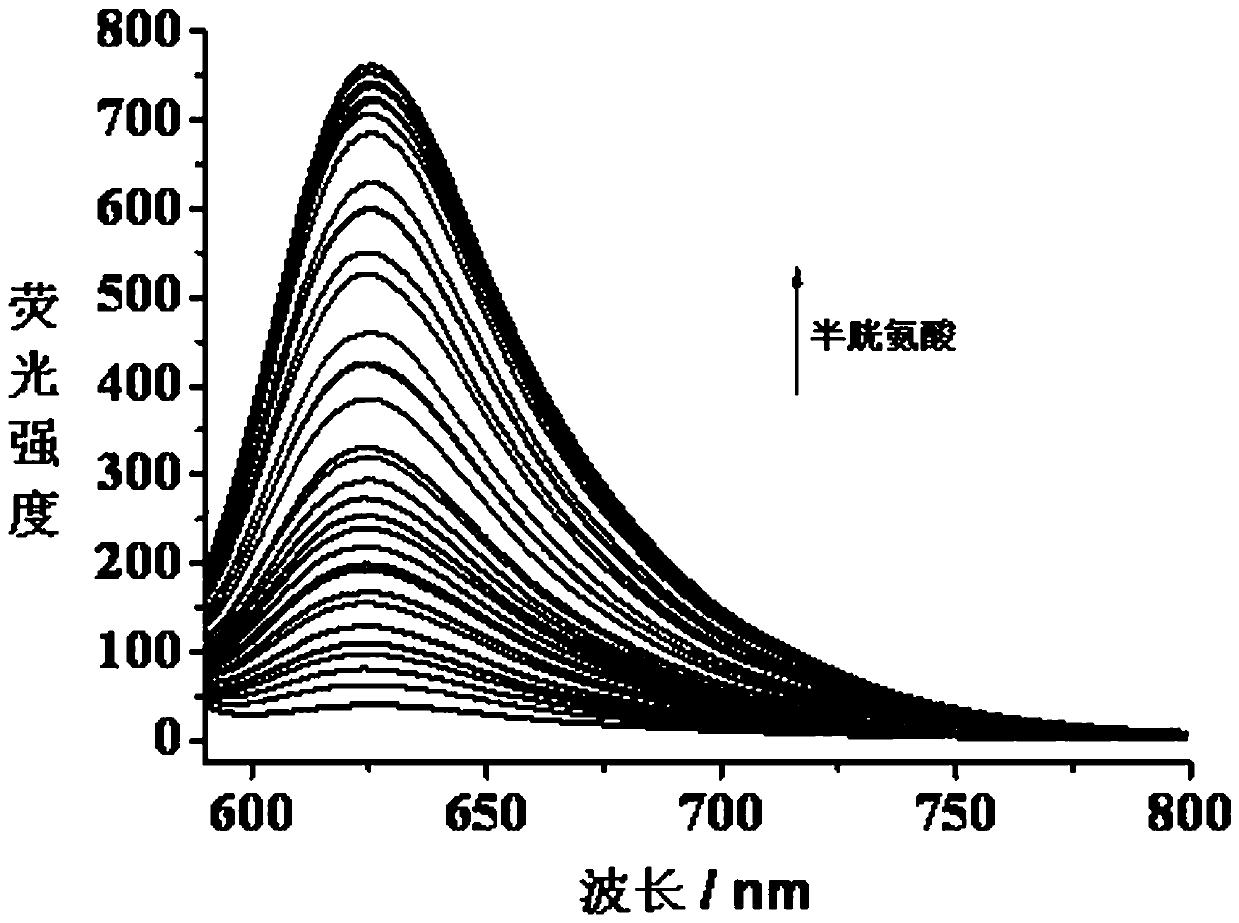
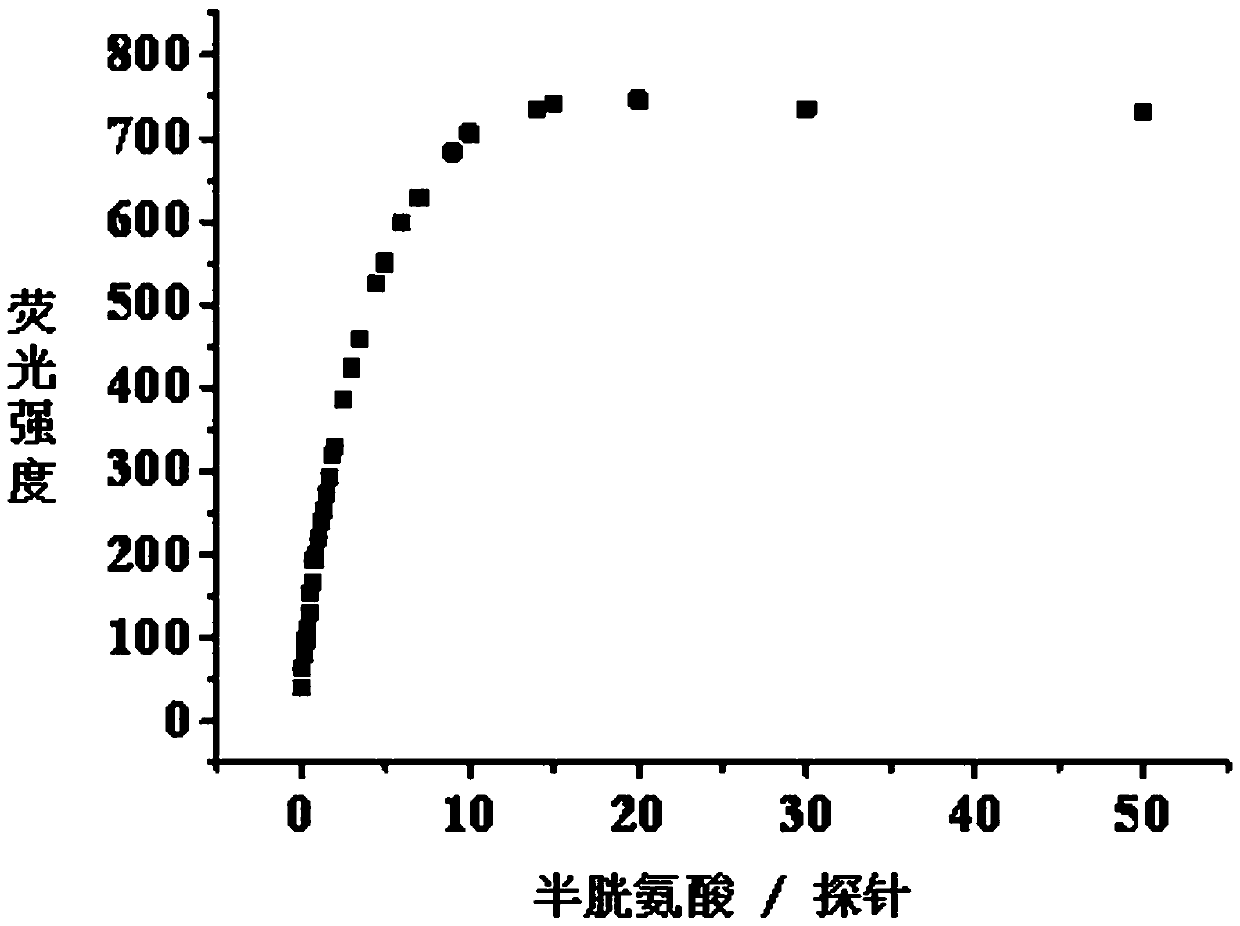
[0032] Embodiment 3: titration experiment of pH=7.4 fluorescent probe and biothiol:
[0033] In the PBS buffer solution with pH=7.4, add the fluorescent probe with an initial concentration of 2 mM, so that the concentration of the fluorescent probe in the solution is 10 μM. Then, different amounts of biothiols with an initial concentration of 20 mM were added sequentially, so that the concentrations of biothiols in the solution were 2 μM, 4 μM, 6 μM, 8 μM, 10 μM, 12 μM, 14 μM, and 16 μM, respectively. , 18 μM, 20 μM, 24 μM, 28 μM, 32 μM, 36 μM, 40 μM, 45 μM, 50 μM, 60 μM, 80 μM, 100 μM, 120 μM, 140 μM, 160 μM, 180 μM, 200 μM, 250 μM, 300 μM, 500 μM, without adding biothiol as a control, let stand for 0.5 hours to fully react biothiol and fluorescent probe. The absorption and fluorescence spectra of different biological thiols were tested with an absorption spectrometer and a fluorescence spectrometer respectively. The excitation wavelength of the fluorescence spectrum was 570...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com