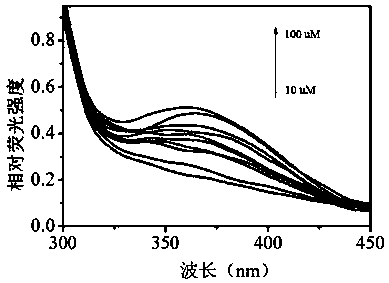
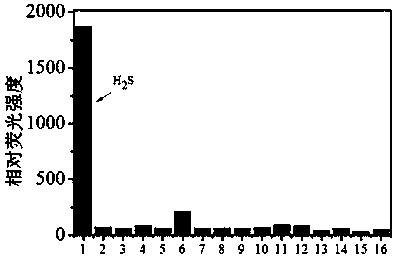
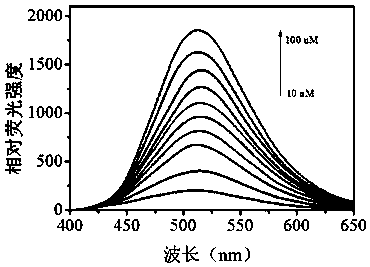
Water-soluble hydrogen sulfide fluorescent probe, preparation method thereof and application thereof in detection of water sulfide and cell hydrogen sulfide
A fluorescent probe and hydrogen sulfide technology, applied in the field of analysis and detection, can solve the problems of poor biocompatibility, high detection cost, and high detection limit, and achieve the effects of good biocompatibility, good water solubility, and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation N-(5-trimethylsilylethynyl quinoline-8-amino) benzamide
[0030]
[0031] 0.1 mmol of N-(5-iodoquinoline-8-amino) benzamide shown in formula I, 0.2 mmol of trimethylsilyl acetylene, 0.04 mmol of bistriphenylphosphine palladium dichloride, 0.015 mmol of CuI, three Add 0.3ml of ethylamine to 15ml of tetrahydrofuran solution, under nitrogen protection, stir and heat up to 50°C for 12h. After the reaction, add saturated aqueous NaCl solution to the reaction solution, extract with ethyl acetate, and dry the organic phase over anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation, and the crude product was separated by column chromatography, using ethyl acetate:petroleum ether as the mobile phase at a ratio of 1:50, to obtain 28 mg of the compound represented by formula II.
[0032] 1 H NMR (400MHz, DMSO) δ10.73(s, 1H), 9.04(dd, J=4.2, 1.5Hz, 1H), 8.73(d, J=8.1Hz, 1H), 8.59(dd, J=8.4, 1.5Hz,1H),8.10–7.99(m,...
Embodiment 2
[0033] Embodiment 2: Preparation of N-(5-trimethylsilylethynyl quinoline-8-amino) benzamide
[0034]
[0035]0.1 mmol of N-(5-iodoquinoline-8-amino) benzamide shown in formula I, 0.3 mmol of trimethylsilyl acetylene, 0.05 mmol of bistriphenylphosphine palladium dichloride, 0.015 mmol of CuI, three Add 0.5ml of ethylamine to 15ml of tetrahydrofuran solution, under nitrogen protection, stir and raise the temperature to 70°C for 12h. After the reaction, add saturated aqueous NaCl solution to the reaction solution, extract with ethyl acetate, and dry the organic phase over anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation, and the crude product was separated by column chromatography, using ethyl acetate:petroleum ether at a ratio of 1:50 as the mobile phase, to obtain 31 mg of the compound represented by formula II.
Embodiment 3
[0036] Embodiment 3: Preparation N-(5-ethynyl quinoline-8-amino) benzamide
[0037]
[0038] Add 0.1mmol of N-(5-trimethylsilylethynylquinoline-8-amino)benzamide represented by formula II and 0.4mmol of KOH into 25ml of methanol solution, stir and heat to 40°C, and react for 12h. , add saturated aqueous NaCl solution to the reaction solution, extract with ethyl acetate, dry the organic phase through anhydrous magnesium sulfate, filter, remove the solvent through rotary evaporation, and separate the crude product through column chromatography, ethyl acetate:petroleum ether is 1:50 As the mobile phase, 18 mg of the compound represented by formula III was obtained.
[0039] 1 H NMR (400MHz, DMSO) δ10.76(s, 1H), 9.06(dd, J=4.2, 1.5Hz, 1H), 8.74(d, J=8.1Hz, 1H), 8.65(dd, J=8.4, 1.5Hz,1H),8.13–8.01(m,2H),7.91(d,J=8.1Hz,1H),7.83(dd,J=8.4,4.2Hz,1H),7.73–7.60(m,3H), 4.68(s,1H). 13 C NMR (101MHz, DMSO) δ165.12, 150.24, 138.26, 135.52, 134.96, 134.63, 132.83, 132.60, 129.56, 128.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com