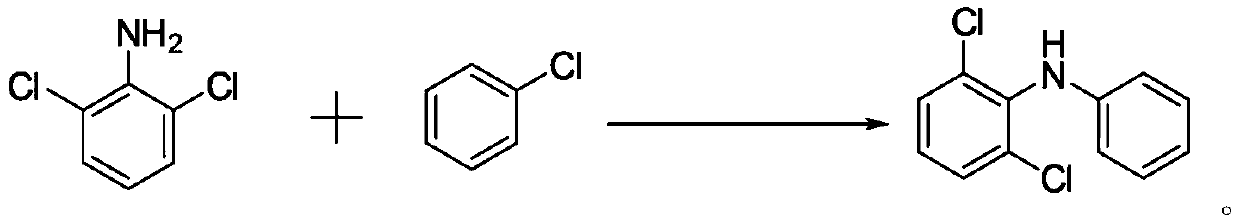
Preparation method of 2, 6-dichlorodiphenylamine
A technology of dichlorodiphenylamine and dichloroaniline, which is applied in the field of chemical drug synthesis, can solve the problems of difficult control of intermediates, high production costs, and many wastes, achieve high unit active sites, reduce reaction costs, and reduce usage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
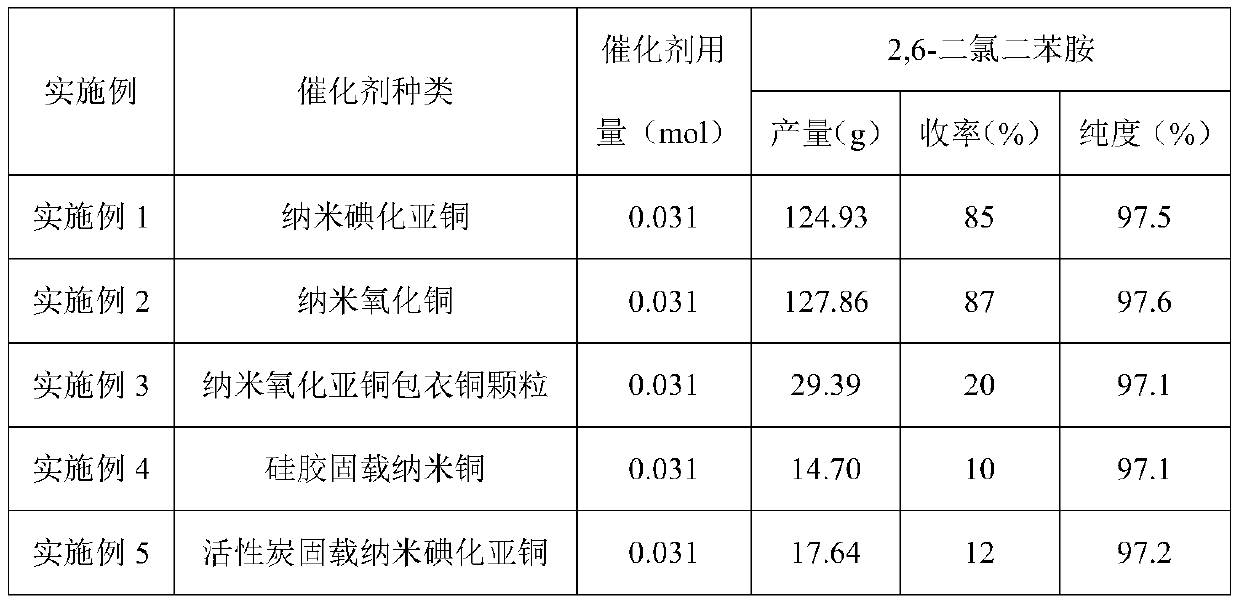
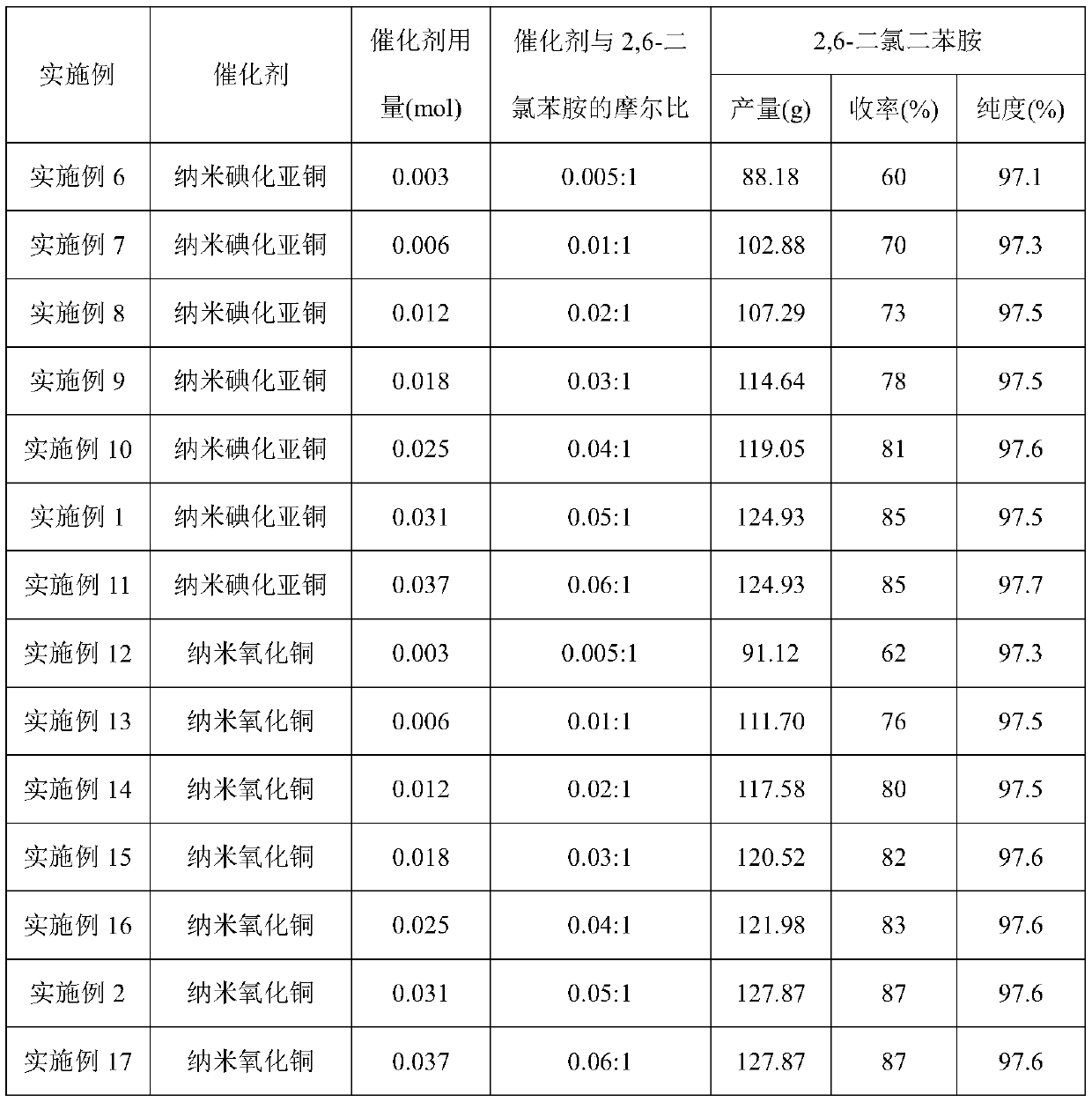
Embodiment 1
[0026] A preparation method for 2,6-dichlorodiphenylamine, comprising the following steps:
[0027] (1) Add 2,6-dichloroaniline (100g, 0.617mol) and N,N-dimethylformamide (600ml) into the reaction flask, stir and dissolve, then add chlorobenzene (347.25g, 3.085mol) , Potassium Phosphate (261.929g, 1.234mol) and catalyst (0.031mol), stir and heat up to 130 ℃, insulation reaction 20h; Journal of Organic Chemistry 2011,76,2296-2300) prepared by the method described.
[0028] (2) After the reaction mixture obtained in step (1) is cooled to 80°C, heat filter with diatomaceous earth, collect the filtrate, wash the filter cake with hot water at 50°C, collect the lotion, combine the filtrate and the lotion, and distill under reduced pressure to remove water, Chlorobenzene and N,N-dimethylformamide to obtain a crude product; the crude product was dissolved in dichloromethane, added water to separate layers, distilled from dichloromethane to recover the solvent, and distilled under red...
Embodiment 2
[0030] The content of Example 2 is basically the same as that of Example 1, except that the catalyst is nano-copper oxide particles, and the nano-copper oxide is purchased from Aldrich.
Embodiment 3
[0032] The content of embodiment 3 is basically the same as embodiment 1, and its difference is: catalyst is nano cuprous oxide coating copper particle, and described cuprous oxide coating nano copper particle is according to document (Chemical Communication 2004,778-779 ) prepared by the method recorded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com