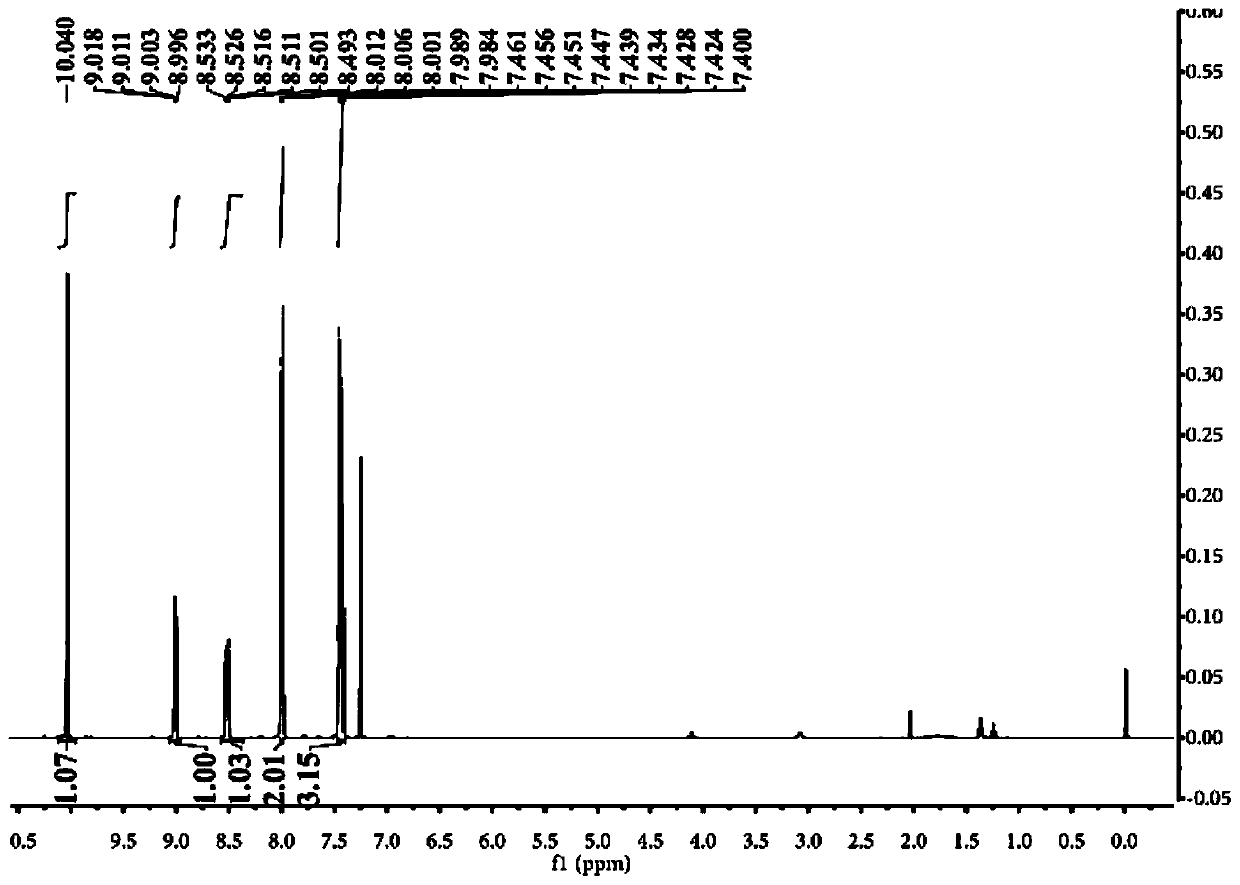
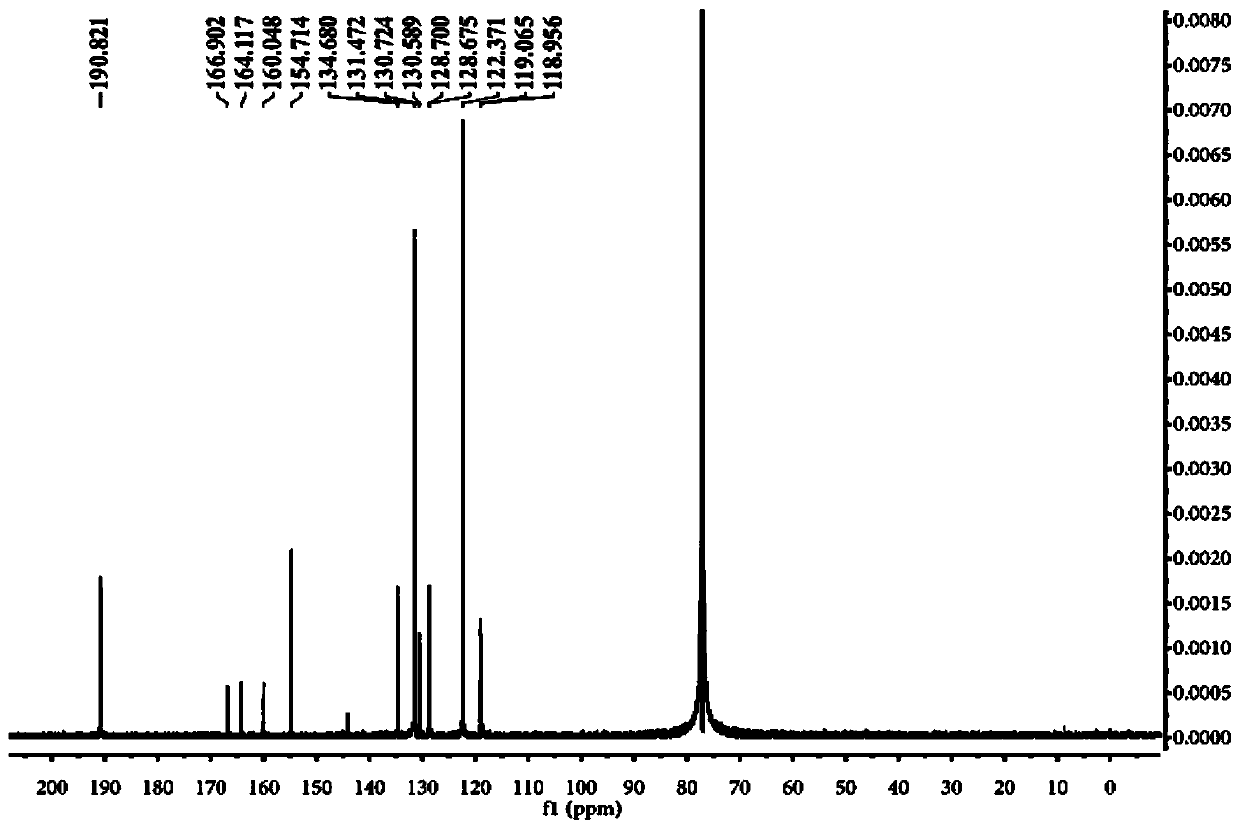
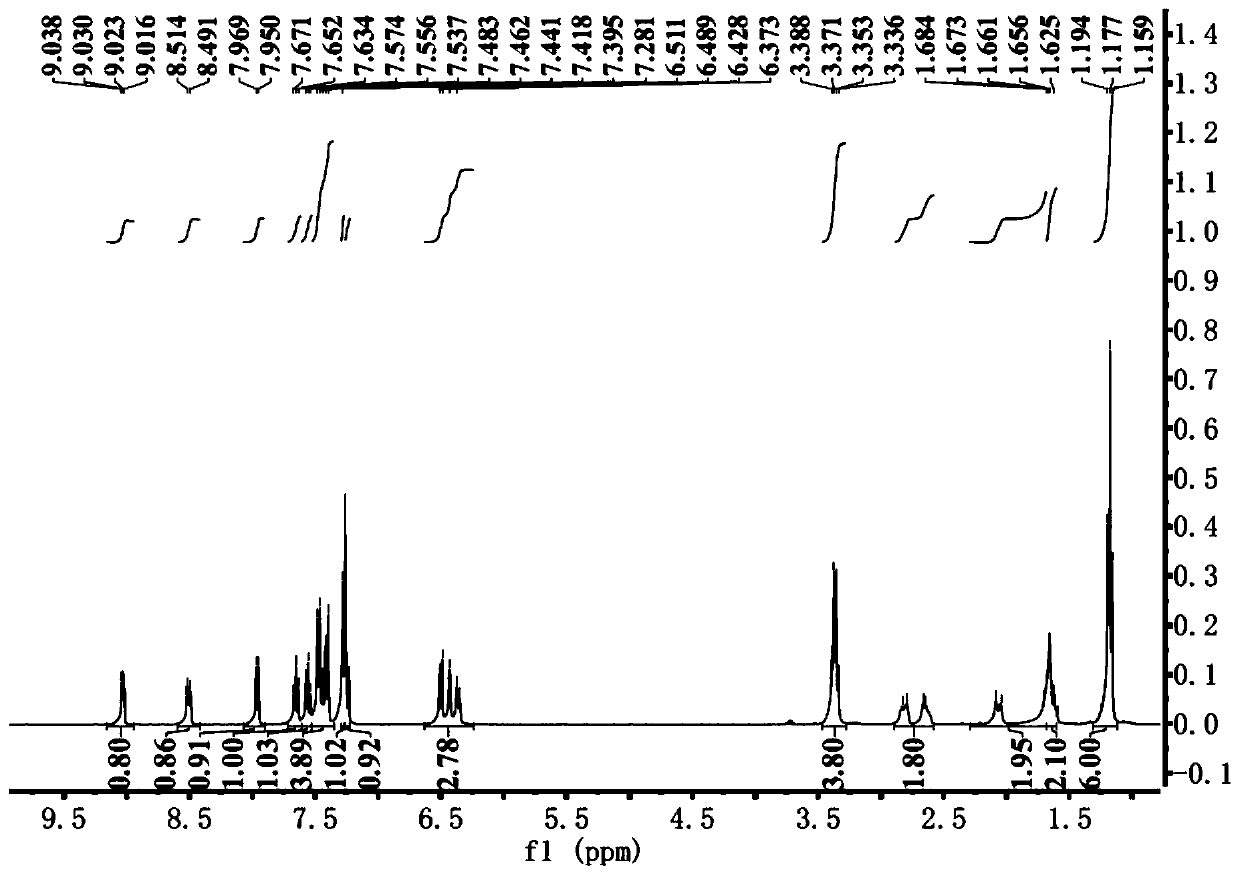
Near-infrared fluorescent probe for hydrogen polysulfides, and preparation method and application of near-infrared fluorescent probe
A fluorescent probe and near-infrared technology, which is applied in the field of chemistry and analysis and detection, can solve the problems of lack of fluorescent probes, detection difficulties, dynamic changes, etc., and achieve the effect of good selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0071] 1. Materials and instruments
[0072] LPS (lipopolysaccharide from Escherichia coli 026B6); MTT cell proliferation / toxicity detection kit (Biosharp); Gibco DMEM high glucose medium (US Life Technologies); Gibco fetal bovine serum (US Life Technologies); penicillin (100 μg / mL) and streptomycin (100 μg / mL) (Life Technologies, USA); TLC using GF 254 Silica gel plate (250 μm), column chromatography using 300-400 mesh silica gel (Qingdao Ocean Chemical); the rest of the reagents are domestic analytical grade.
[0073] cell:
[0074] Species and strains: human breast cancer MCF-7 cell line.
[0075] Experimental animals:
[0076] Species and strains: healthy male Kunming mice, weighing 20-25 g. Source: Experimental Animal Center of Xuzhou Medical University.
[0077] instrument:
[0078] ECZ-400S nuclear magnetic resonance instrument (Japan JEOL company); FV1000 laser confocal scanning microscope (Olympus, Japan); F4600 fluorescence spectrophotometer (Hitachi, Japan); ...
Embodiment 1
[0109] Preparation of Compound Ⅰ: Under ice-bath conditions, cyclohexanone (508 μL, 4.92 mmol) was slowly added dropwise to concentrated sulfuric acid (6.7 mL, 125.05 mmol), and 4-diethylamino ketoacid (0.5 g, 1.59 mmol). The mixed solution was reacted at 90° C. for 2 h, cooled to room temperature, and poured into ice water. Perchloric acid (620 μL, 10.86 mmol) was slowly added, and a red solid was precipitated, which was filtered by suction and washed with water (3×20 mL) to obtain 369.81 mg of compound with a yield of 48.9%. TLC (silica, CH 2 Cl 2 :CH 3 OH, 20:1v / v): R f = 0.4.
Embodiment 2
[0111] Preparation of 2-fluoro-5-nitrobenzoyl chloride: 2-fluoro-5-nitrobenzoic acid (0.5 g, 2.70 mmol) was dissolved in thionyl chloride (20 mL), and refluxed at 80° C. for 5 h. Distillation under reduced pressure gave 0.49 mg of solid, with a yield of 90.7%. TLC (silica, CH 2 Cl 2 :CH3 OH, 20:1v / v): R f = 0.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com