Pentacetylgardenoside cyclohexanamide capable of reducing uric acid activity and preparation method and application of pentacetylgardenoside cyclohexanamide
A technology of pentaacetylgardenoside cyclohexanamide and triethylamine, which is applied in the field of pentaacetylgardenoside cyclohexanamide and its preparation, can solve problems such as urate crystal deposition and renal function damage, and improve biological activity , enhanced inhibitory activity, novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
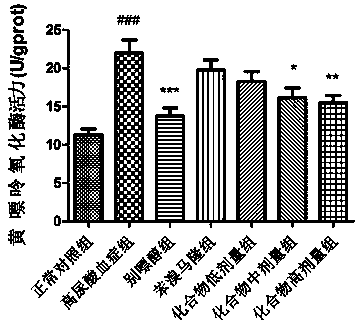
[0034] Add 388mg (1mmol) of geniposide and 4mL of 6% (mass fraction) NaOH solution into a 25mL round bottom flask equipped with a reflux device, heat to 60°C and carry out hydrolysis reaction while stirring, TLC traces the reaction process [developing solvent V (ethanol): V (methanol) = 5:1], the reaction time is about 2 hours, after the reaction is completed, the reaction solution is evaporated to dryness to obtain gardeniaic acid.
Embodiment 2
[0036]
[0037] Add 374mg (1mmol) of gardenic acid (1mmol) and 111mg (1.1mmol) of triethylamine to a 50mL round-bottomed flask, mix and stabilize for 15min under stirring, then slowly add 122mg (1.2mmol) of acetic anhydride to the round-bottomed flask dropwise, After the dropwise addition, the esterification reaction was carried out at room temperature, and the reaction process was tracked by TLC [developing agent: V (petroleum ether): V (ethyl acetate) = 1:1, 1 drop of formic acid], after the reaction was completed, use 5 mL of dichloromethane and The reaction solution was extracted twice with 5 mL of saturated sodium bicarbonate solution, and the organic phases were combined and evaporated to dryness to obtain pentaacetylgardenic acid.
[0038] 1 H NMR (400 MHz, DMSO) δ 7.10 (s, 1H), 5.78 (s, 1H), 5.32 (t, J = 9.7Hz, 1H), 5.12 (d, J = 8.1 Hz, 1H), 5.00 (d, J = 5.4 Hz, 1H), 4.90 (t, J = 9.8Hz, 1H), 4.77 (dd, J = 9.7, 8.2 Hz, 1H), 4.63 (s, 2H), 4.16 (dd, J = 12....
Embodiment 3
[0041]
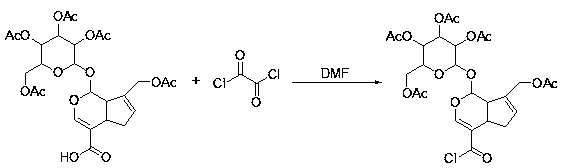
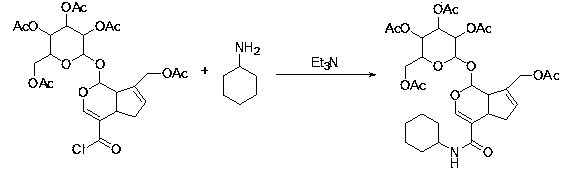
[0042]In a 50 mL round bottom flask, 584 mg (1 mmol) of the obtained pentaacetylgardenic acid was dissolved in 5 mL of dichloromethane, 0.5 mL of DMF was added in an ice-water bath, and it was stirred for 15 minutes to stabilize, and then oxalyl chloride solution 191 mg of oxalyl chloride was slowly added dropwise ( 1.5mmol), after the dropwise addition, carry out the acid chloride reaction at room temperature, the reaction time is about 2 hours, after the reaction is completed, the solvent and excess oxalyl chloride are evaporated to dryness (under anhydrous conditions) to obtain pentaacetyl geniposide chloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com