Three-dimensional spherical α-helical cationic polypeptide with high-efficiency gene delivery ability, its preparation method and application
A technology of gene delivery and cationic polymerization, which can be used in gene therapy, genetic non-effective components, and non-effective components of polymer compounds. , the effect of efficient gene transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Dissolve L-glutamic acid in water (375 mL) and heat to 70 °C with stirring, then add aqueous solution (375 mL) of hydrated copper acetate (18.6 g, 103 mmol) to L-glutamic acid in solution. After that, the stirring was stopped, and after standing at room temperature for 48 h, the precipitate was stirred and washed with water, ethanol, and petroleum ether for 24 h, and the blue solid was obtained by suction filtration, that is, copper (II) L-glutamate complex, which was freeze-dried Store in a desiccator for later use;
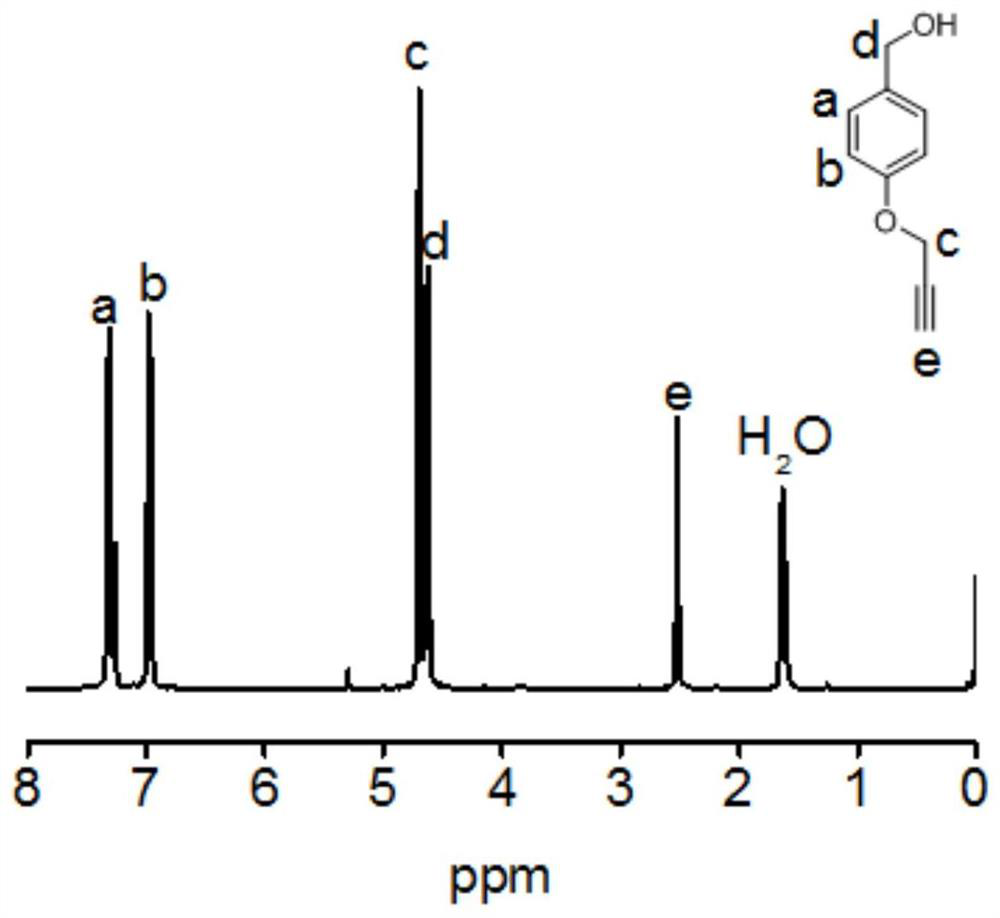
[0097] Potassium carbonate (15.2 g, 0.11 mol) and p-hydroxybenzyl alcohol (9.3 g, 0.075 mol) were dissolved in acetone (150 mL), and propyne bromide (6.75 mL, 0.09 mol) and 18-crown Ether-6 (0.1 g). After the solution was refluxed at 75 °C for 12 h, the acetone was removed by a rotary evaporator, and water (200 mL) was added to dissolve the remaining solid. The solution was extracted with dichloromethane (30 mL×3), and the organic phases were combined....
Embodiment 2
[0102] 1, 6-Dibromohexane (1.26 mL, 8 mmol) and sodium azide (1.6 g, 24 mmol) were dissolved in DMF (19 mL), and reacted at 60 °C for 24 h. Then water (150 mL) was added to dissolve the insoluble matter, extracted with diethyl ether (20 mL×3) and the organic phase was collected. After the organic phase was dried with sodium sulfate, it was filtered and rotary evaporated to obtain a white oily substance, that is, compound 4, which was subjected to NMR with deuterated chloroform. Figure 5 its NMR spectrum.
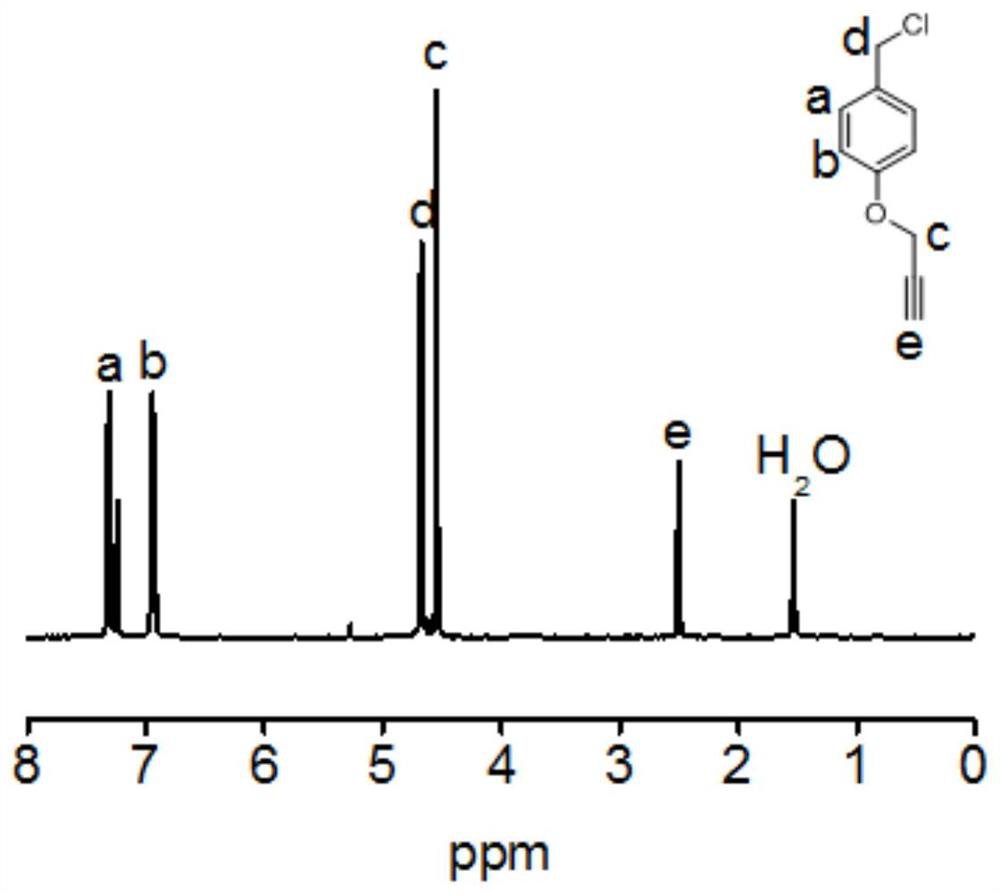
[0103] Compound 4 (3.33 g, 20 mmol) was dissolved in a mixed solvent of diethyl ether (15 mL) and ethyl acetate (15 mL), and 5 % hydrochloric acid solution (30 mL) was added, and triphenyl Phosphorus (5.51 g, 22 mmol), ensured that the two phases were separated and stirred slowly for 1 h, then reacted at room temperature for 24 h. Subsequently, 1M hydrochloric acid solution (30 mL) was added to wash the organic phase, and the aqueous phase was collected after separation. ...
Embodiment 3
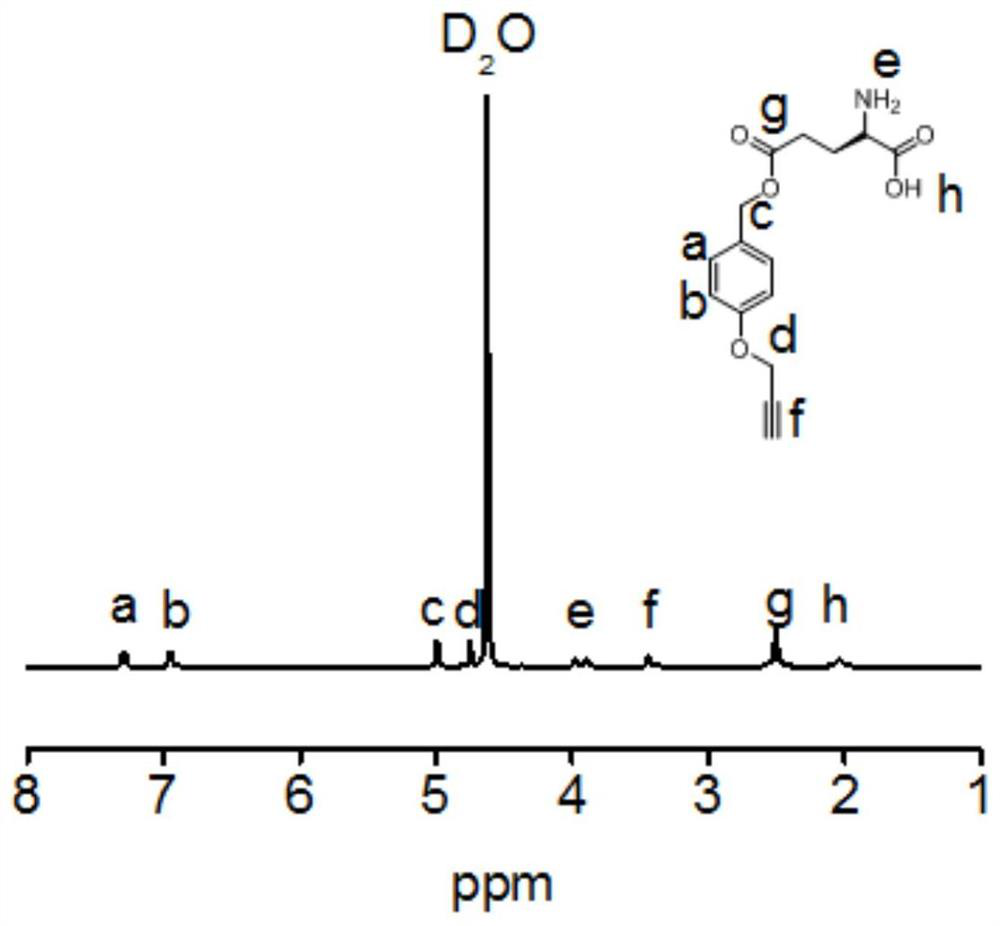
[0106] In a glove box, dissolve γ-(4-propargyloxybenzyl)-L-glutamic acid-N-carboxylic acid anhydride monomer (50 mg, 1.6 mmol) in anhydrous N,N-dimethylformaldehyde Amide (1 mL), and the third-generation dendrimer (0.68 mg, 0.001 mmol) was added, and the reaction was stirred at room temperature for 72 h. Subsequently, the reaction phase was added dropwise into ice methanol (50 mL) to precipitate, and the methanol was removed after centrifugation to obtain polymer A, which was subjected to NMR with deuterated chloroform. Figure 8 its NMR spectrum.
[0107] Dissolve γ-(4-propargyloxybenzyl)-L-glutamic acid-N-carboxylic anhydride monomer (50 mg, 1.6 mmol) in anhydrous dichloromethane (1 mL) in the glove box , and the third-generation dendrimers (0.68 mg, 0.001 mmol) were added, and the reaction was stirred at room temperature for 30 min. Subsequently, the reaction phase was added dropwise into ice methanol (50 mL) to precipitate, and the methanol was removed after centrifugati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com