Fluoro dithio phosphate compound and preparation method and application thereof
A technology of fluorinated phosphorodithioate and phosphorodithioate, which is applied in the field of preparation of positron emission imaging agents, can solve problems such as poor stability, defluorination, and large molecular weight, and achieve high stability The effects of high stability, short labeling time, and low molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
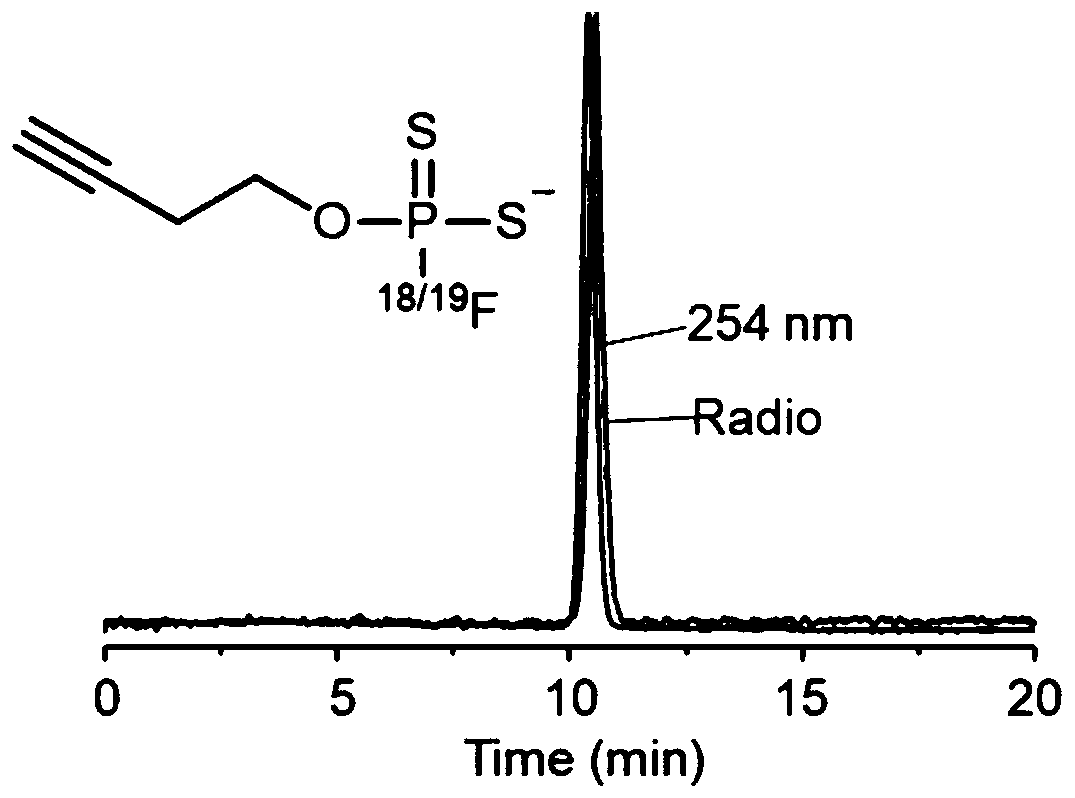
[0080] This embodiment provides a 19 F-labeled fluorodithiophosphate compounds, the preparation process and specific steps are as follows:
[0081]
[0082] Synthetic steps of compound 1:
[0083] Put 10mmol of dichloro(diethylamino)phosphine in the reaction flask, add 40mL of anhydrous benzene under the protection of argon, then add 10mmol of 1,2-ethanedithiol dropwise into the reaction flask under ice-water bath conditions After reacting for 30 minutes, react at room temperature for 2 hours, filter, and spin dry under reduced pressure.
[0084] NMR data of compound 1:
[0085] 31 P NMR (162MHz, CDCl 3 ):δ106.98.
[0086] Synthetic steps of compound 2:
[0087]Put 5mol of compound 1 and 6mmol of 5-ethylthiotetrazole in a reaction flask, add 40mL of anhydrous dichloromethane, add 5mmol of 3-butyn-1-ol, react at room temperature for 3 hours and then filter. Spin dry under reduced pressure.
[0088] NMR data of compound 2:
[0089] 31 P NMR (162MHz, CDCl 3 ): δ148....
Embodiment 2
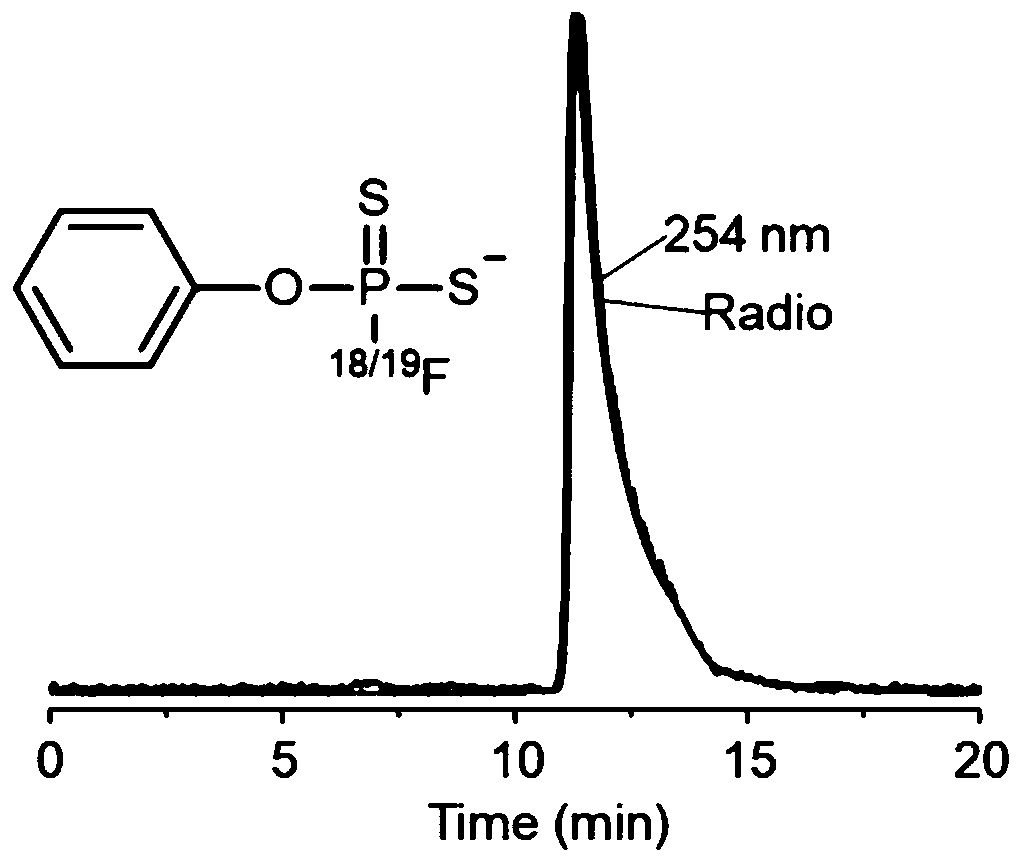
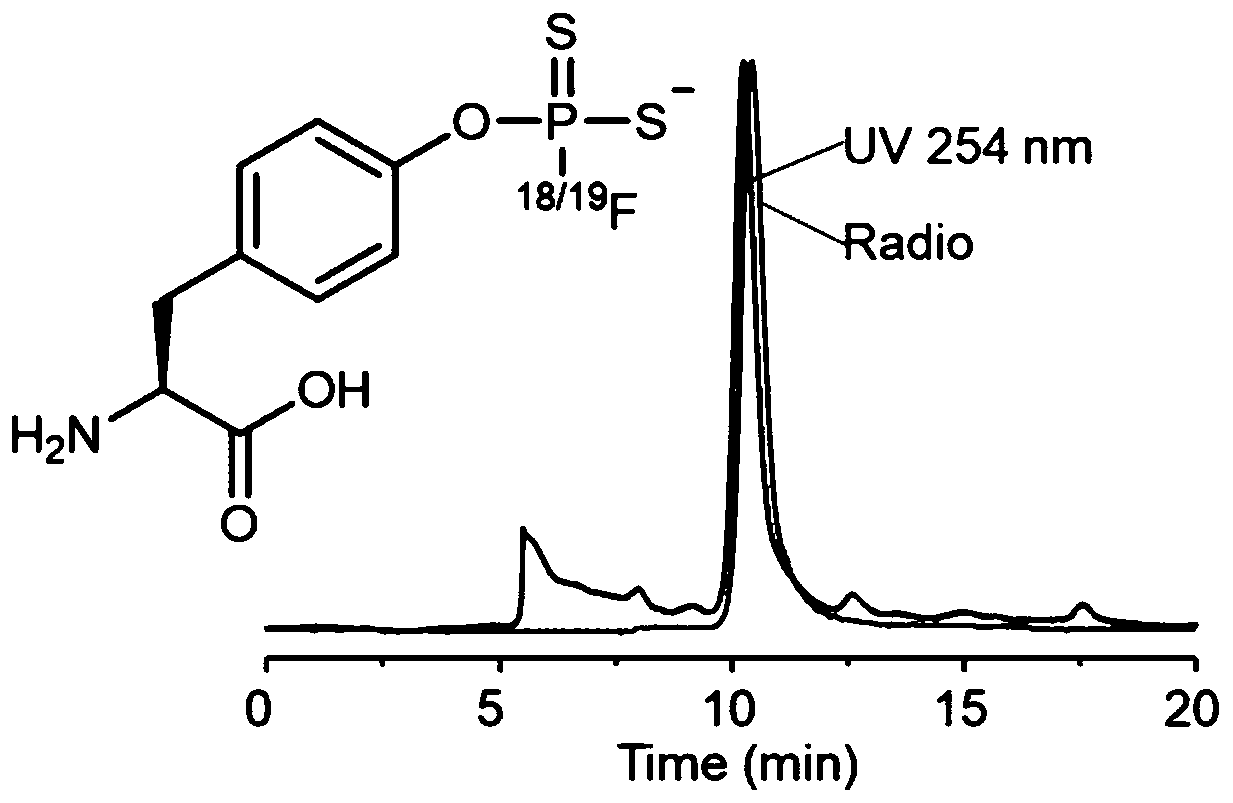
[0099] This embodiment provides a positron emission imaging 18 F-labeled fluorodithiophosphoric ester compounds, the synthesis steps of compound 3 are the same as in Example 1, and the specific steps of its labeling are as follows:
[0100]
[0101] Method 1: Use 8mg K2.2.2 and 1mg K 2 CO 3 Acetonitrile water rinse [ 18 F]F - Enriched QMA-Sep-Pak column, after acetonitrile azeotropic water removal, use K 2 CO 3 , K2.2.2, [ 18 F]F - The mixture and 0.5 mg of compound 3 were dissolved in 100 μL of acetonitrile solvent, and reacted at 20° C. for 30 seconds. Stop the reaction, add about 10mL water to dilute the reaction system, then pass through the Sep-Pak C18 column, and collect the filtrate in No. 1 bottle (mainly the [ 18 F]F - ), and then wash the column with 10mL of water, and collect the filtrate into bottle No. 2 (make sure that [ 18 F]F - Thoroughly rinsing clean), dry the Sep-Pak C18 column with nitrogen, wash the C18 column with 2mL of acetonitrile, collec...
Embodiment 3
[0102] Embodiment 3 (product structure is with embodiment 2)
[0103] Method 2: with [ 18 F]F - The fluoride ion aqueous solution and 0.5 mg of compound 3 were dissolved in 100 μL of acetonitrile solvent, and reacted at 20° C. for 30 seconds. Stop the reaction, add about 10mL water to dilute the reaction system, then pass through the Sep-Pak C18 column, and collect the filtrate in No. 1 bottle (mainly the [ 18 F]F - ), and then wash the column with 10ml of water, and collect the filtrate into bottle No. 2 (make sure that the [ 18 F]F - Thoroughly rinsing), dry the Sep-Pak C18 column with nitrogen, wash the Sep-Pak C18 column with 2mL of acetonitrile, collect the filtrate into No. Acetonitrile was dried to obtain 18 F-labeled fluorodithiophosphate compounds [ 18 F] 4, the radiochemical purity of the marker is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com