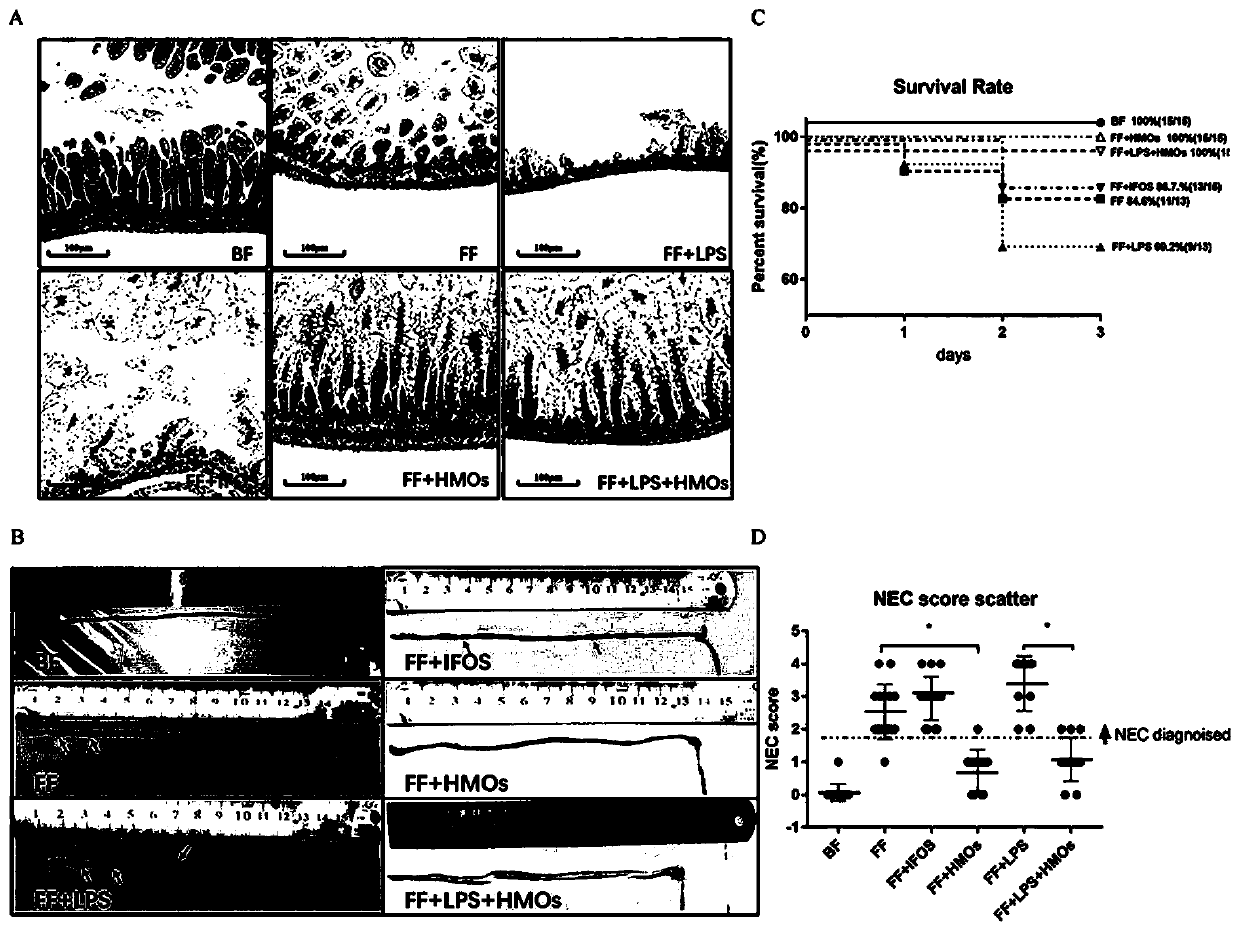
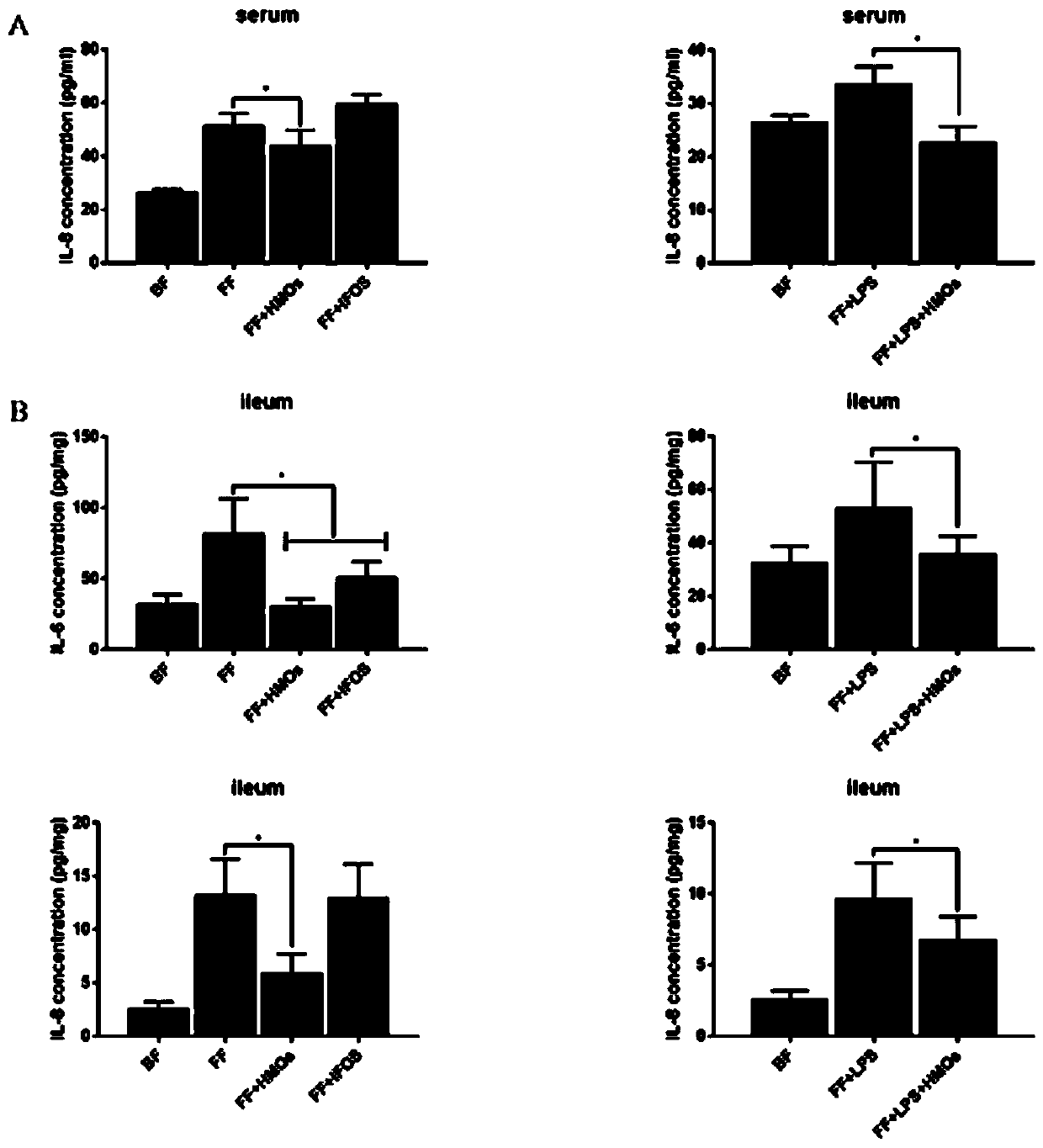
Human milk oligosaccharides and application thereof in preparation of medicines for treating or preventing NEC by relieving intestinal tract hypoxia injury
A technology for human milk oligosaccharides and uses, applied in the preparation of sugar derivatives, dairy products, sugar derivatives, etc., can solve the problems of low content and structure complexity, unclear mechanism of action, and limited wide application, etc. Apoptosis, preventing morbidity, reducing the effect of cellular inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
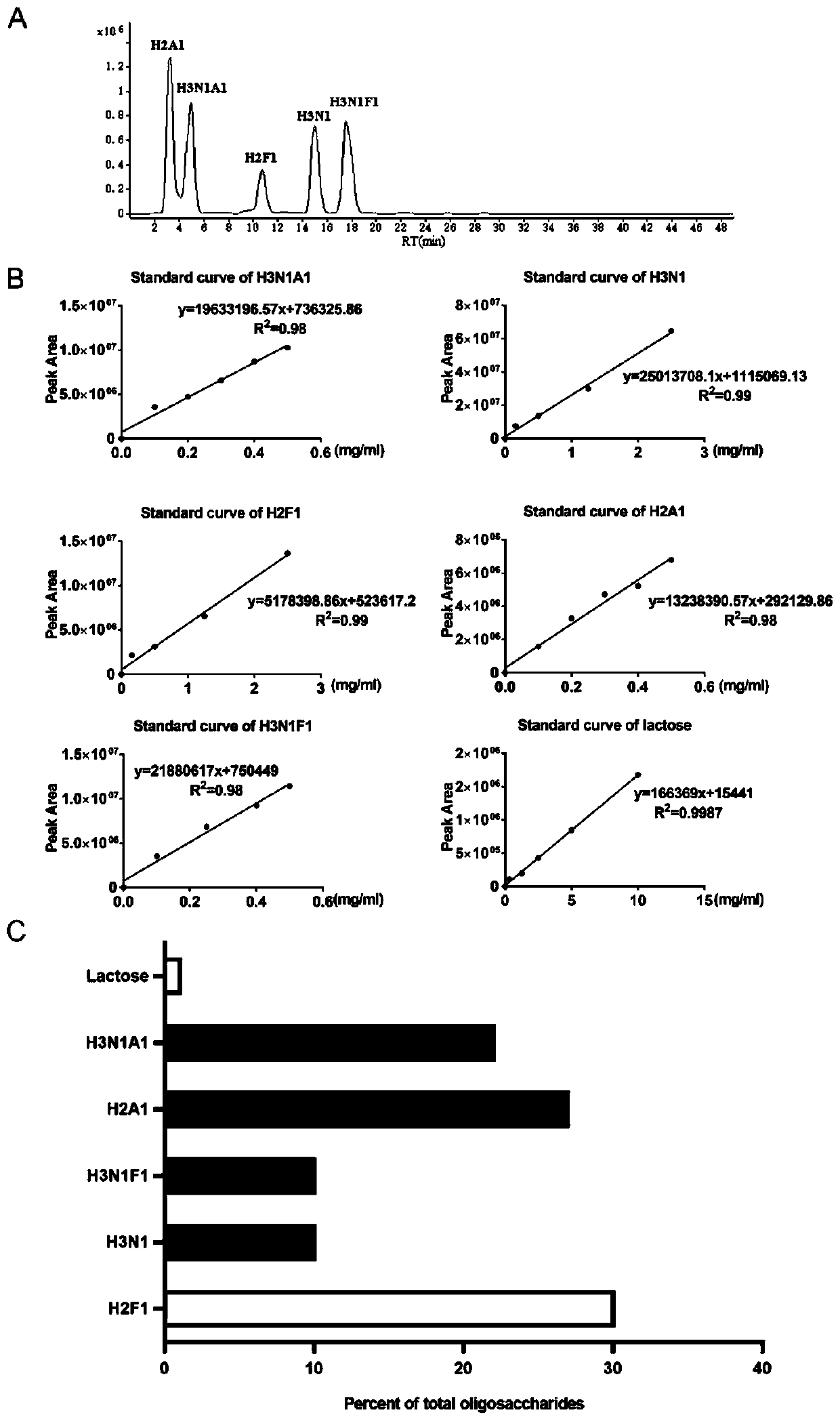
[0030] This embodiment provides a kind of human milk oligosaccharide, the preparation method of described human milk oligosaccharide is:
[0031] Mix 10L of human milk evenly, and centrifuge at 14000g for 30min at 4°C to remove fat. The collected whey is ultrafiltered with a 10kd ultrafiltration membrane to remove protein and residual fat. The filtrate was treated with 2 volumes of absolute ethanol, and precipitated overnight at 4°C. After centrifuging at 12000g to remove the precipitate, the finally collected supernatant was freeze-dried to obtain crude oligosaccharides. Crude oligosaccharides were separated by Luna HILIC column (10×250mm, 5μm, Phenomenex) to remove lactose, chromatographic conditions: A liquid ultrapure water, B liquid chromatography pure acetonitrile, 0–40min, 80%–50%B; 40–45min , 20% B; 45–55min, 80% B. One tube of filtrate was recovered every minute, and the filtrate was identified by HPLC-MS / MS-QTOF (Agilent 6545, USA). , Phenomenex), the chromatograp...
Embodiment 2
[0076] This embodiment provides an infant formula milk powder added with human milk oligosaccharides, its formula is: protein 10g / 100g, fat 28g / 100g, carbohydrate 52g / 100g, taurine 34mg / 100g, L-carnitine 7.5mg / 100g, inositol 32mg / 100g, β-carotene 61mg / 100g, lutein 100μg / 100g, calcium 380mg / 100g, phosphorus 224mg / 100g, magnesium 40mg / 100g, sodium 144mg / 100g, potassium 625mg / 100g, chlorine 350mg / 100g, Zinc 4.5mg / 100g, Iron 5.4mg / 100g, Copper 400μg / 100g, Manganese 100μg / 100g, Iodine 100μg / 100g, Selenium 12.3μg / 100g, Vitamin A190 IU / 100g, Vitamin D 380 IU / 100g, International Units of Vitamin E / 100g, Vitamin K 1 54μg / 100g, vitamin C 80mg / 100g, vitamin B 1 660μg / 100g, Vitamin B 2 1100μg / 100g, Vitamin B 6 400μg / 100g, Vitamin B 12 1.5μg / 100g, niacin 5000μg / 100g, pantothenic acid 2600μg / 100g, folic acid 76μg / 100g, biotin 20μg / 100g, choline 80mg / 100g, nucleotide 58mg / 100g, energy 510kcal / 100g. The human milk oligosaccharides used are the human milk oligosaccharides prepared b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com