Preparation method and intermediate of isoquinoline compound
A technology for compounds and intermediates, which is applied in the field of preparation of isoquinolinic acid compounds, key intermediates of roxacus, a drug for treating chronic anemia, can solve the problems of long overall route, difficulty in process amplification, low total yield and the like, and achieve product The effect of high purity, high yield and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
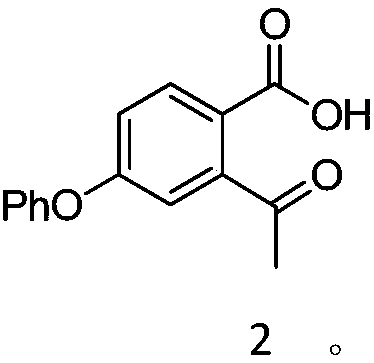
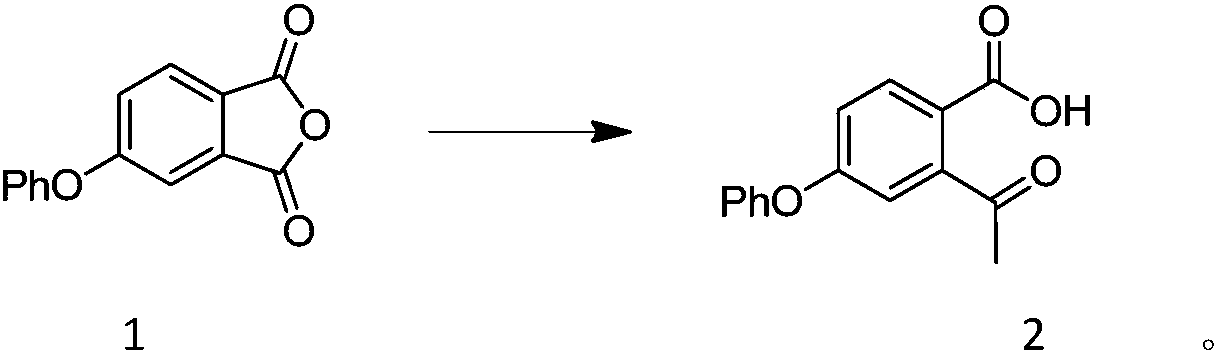
[0044] Add compound formula 1 (24.02g, 100mmol) and tetrahydrofuran (120mL) into the three-necked flask, stir to dissolve, cool to -10~0°C and switch nitrogen for 3 times in vacuum, under the protection of nitrogen, methylmagnesium chloride tetrahydrofuran solution (2.0M, 55mL ) slowly drop into the reaction bottle, and keep the reaction for 2 to 4 hours after dropping. Add 1mol / L dilute hydrochloric acid (240mL) to quench the reaction at the end of the reaction, extract the aqueous phase twice with ethyl acetate (120mL), combine the organic phases and wash once with saturated brine (120mL), dry over anhydrous sodium sulfate, concentrate and use petroleum Compound 2 (21.78 g, 85%) was isolated by recrystallization from a mixed solvent of ethyl ether acetate. MS(EI)m / z=256.0 1 H NMR (400MHz, CDCl 3 )δ7.83(d, J=8.0Hz, 1H), 7.47(q, J=7.7Hz, 2H), 7.31(d, J=6.8Hz, 1H), 7.14(d, J=7.8Hz, 3H) , 7.09 (s, J=12.6Hz, 1H), 1.93 (s, 3H).
[0045] Here, methylmagnesium chlori...
Embodiment 2
[0047]
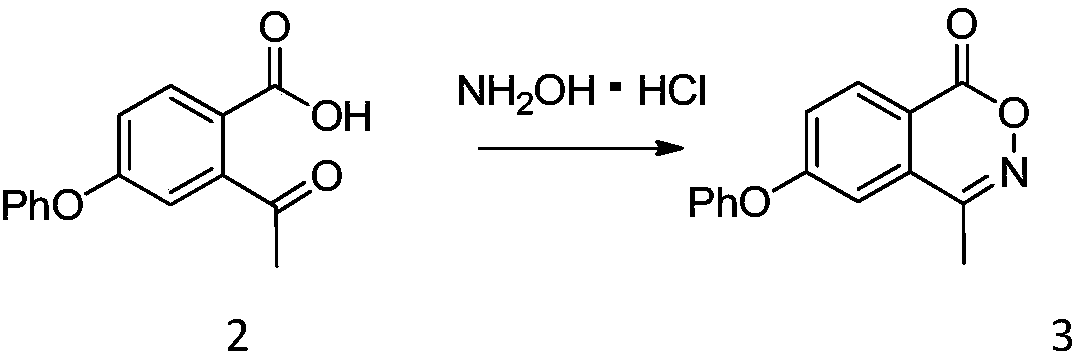
[0048] Add compound 2 (25.63g, 100mmol) and isopropanol (128mL) into a three-neck flask, add potassium carbonate (20.73g, 150mmol), stir well, add hydroxylamine hydrochloride (8.34g, 120mmol) slowly, and heat to 75-80°C React for 10-16 hours. At the end of the reaction, cool the reaction solution to 0-5°C, slowly add water to quench the reaction, remove most of the isopropanol by rotary evaporation, cool to 0-5°C for beating for 1 hour, filter, and use a mixed solvent of isopropanol and water for the crude product Compound 3 (22.79 g, 90%) was obtained by recrystallization. MS(ESI)m / z=254.1[M+H] +1 H NMR (400MHz, CDCl 3)δ8.30(d, J=8.7Hz, 1H), 7.47(t, J=7.9Hz, 2H), 7.34(dd, J=8.7, 2.3Hz, 1H), 7.29(m, J=12.6, 5.1 Hz, 1H), 7.13 (d, J=2.3Hz, 2H), 7.11 (s, 1H), 2.47 (s, 3H).
[0049] Here potassium carbonate can be replaced by sodium carbonate, potassium hydroxide or sodium hydroxide; isopropanol can be used methanol, ethanol, acetone, dichloromethane, 1,2-dichloroe...
Embodiment 3
[0051]
[0052] Add compound 3 (25.33g, 100mmol), phosphine reagent 4a (41.81g, 120mmol) and toluene (128mL) into the three-necked flask, add N,N-diisopropylethylamine (19.39g, 150mmol), heat and reflux reaction 2 ~3 days. At the end of the reaction, cool the reaction solution to 0-5°C, slowly add water (253mL) to quench the reaction, spin off most of the solvent, add ethanol (51mL), filter, and recrystallize the crude product with a mixed solvent of ethanol and petroleum ether to obtain the compound 9a (29.75 g, 92%).
[0053] Here N,N-diisopropylethylamine can be replaced by triethylamine, pyridine, 2,6-lutidine, DBU or potassium tert-butoxide; toluene can be replaced by N,N-dimethylformamide, N,N -Dimethylacetamide, N-methylpyrrolidone, DMPU, xylene or chlorobenzene instead.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com