Drug carrier system based on fluorescence resonance energy transfer of carbon dots and hollow manganese dioxide
A technology of fluorescence resonance energy and manganese dioxide, which is applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve problems that are difficult to remove, affect life activities, and affect the effect of treatment, so as to improve drug utilization and reduce Antioxidant, reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
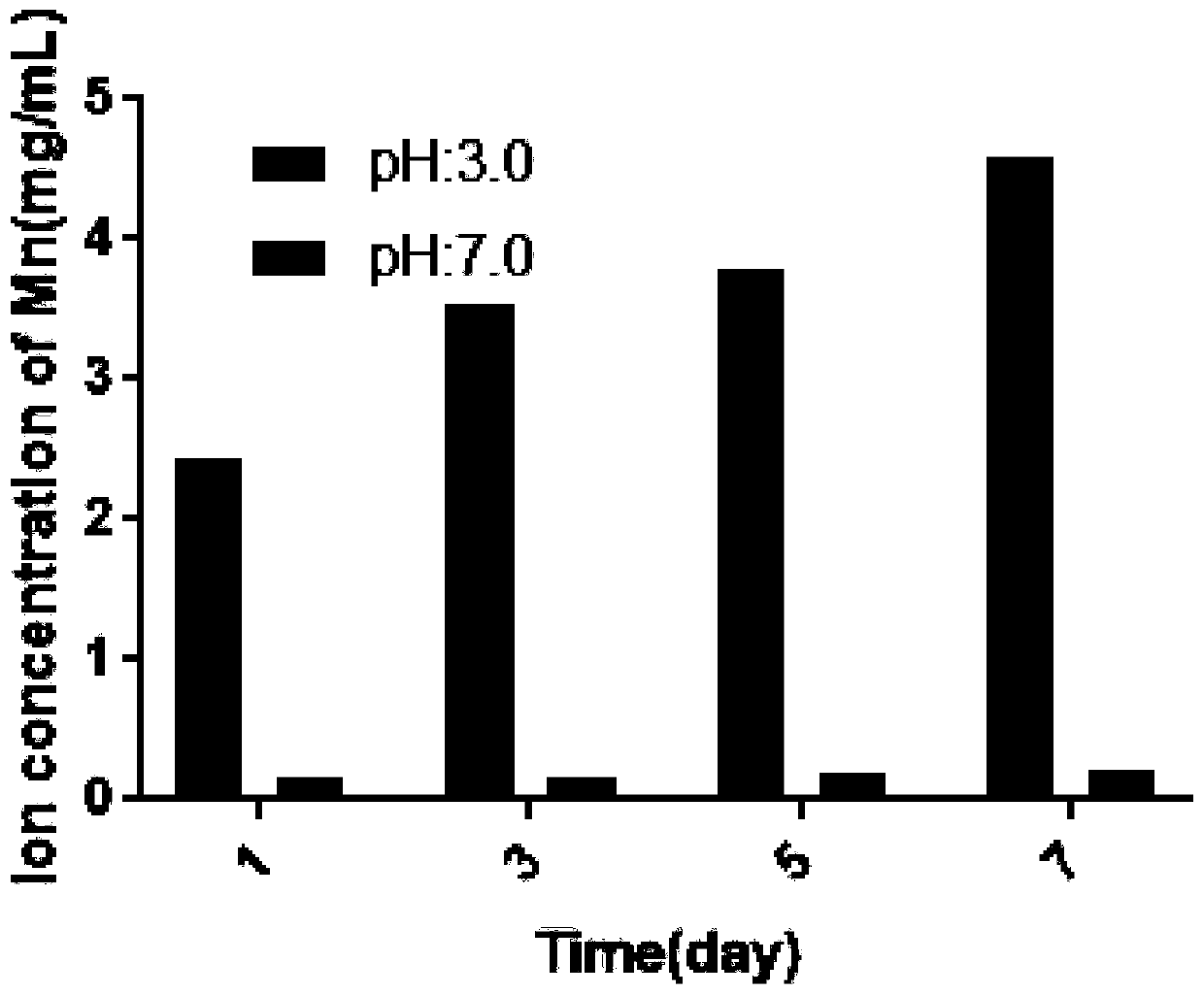
Image
Examples
preparation example Construction
[0042] (2). Preparation of manganese dioxide with core and template:
[0043] Measure 50-80mL of deionized water, 50-80mL of ethanol and 1-3mL of ammonia water, mix well, add 500-1000mg of cetyltrimethylammonium bromide (CTAB) into it, ultrasonically disperse, and at the same time Weigh 100-400 mg of solid nano-silicon spheres obtained in step (1), ultrasonically disperse them into 30-60 mL of deionized water, add the aqueous solution of solid nano-silicon spheres to the above mixed solution, stir at room temperature for 30 minutes, and then add 100- 400mg potassium permanganate (KMnO 4 ), continue stirring at room temperature for 5-12 hours, then centrifuge (10000rpm / min×10min) to precipitate, wash with deionized water and ethanol repeatedly three times, and vacuum-dry for later use.
[0044] (3). Remove the solid nano-silicon balls:
[0045] Redisperse 300-500 mg of the product in step (2) into 20-80 mL of deionized water, add 500-1500 mg of sodium carbonate (Na 2 CO 3 )...
Embodiment 1
[0054] A drug carrier system based on fluorescence resonance energy transfer of carbon dots and hollow manganese dioxide, the specific steps are as follows:
[0055] Step 1. Preparation of blue light carbon dot CDs
[0056] Weigh 1.0507g of citric acid (CA) and ultrasonically disperse it into 10mL of deionized water, add 335 microliters of ethylenediamine to the above solution with a pipette gun, then transfer the solution to a 20mL polytetrafluoroethylene liner, Put it into a 20mL polytetrafluoroethylene autoclave, react at 200°C for 5h, wait for it to cool naturally, use a dialysis bag with a molecular weight of 1000 for two days, and then vacuum dry the product at 60°C for 12h after rotary evaporation to obtain Blue light carbon quantum dots.
[0057] Depend on Figure 4 It can be seen that the optimal emission spectrum of the blue-light carbon quantum dots provided in this embodiment is 400-450 nm.
[0058] Step 2, preparation of hollow manganese dioxide (H-MnO 2 )
...
Embodiment 2
[0084] A drug carrier system based on fluorescence resonance energy transfer of carbon dots and hollow manganese dioxide, the specific steps are as follows:
[0085] Step 1, branched polyethyleneimine (PEI) coated hollow manganese dioxide (H-MnO2):
[0086] First prepare 300mL0.01mol / L Tris HCl buffer solution and adjust its pH to 7.4; then weigh 2000mgPEI and dissolve it in 50mL Tris HCl buffer solution, and after ultrasonic dispersion, obtain PEI solution for use; weigh 200mgH-MnO 2 Ultrasonic disperse into 300mL Tris HCl buffer solution, add 100mgDOX in a dark environment, and stir at room temperature for 24h. After the reaction was completed, the stirring was accelerated, and the PEI solution was slowly added dropwise to the H-MnO 2 In the solution, after all the PEI solution is added dropwise, adjust the pH of the system to 7.4, stir rapidly at room temperature for 4 hours, then centrifuge (10000rpm / min×10min) to precipitate, wash repeatedly with deionized water and etha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com