Mutated chitinase and application thereof
A technology of chitinase and chitin, which is applied in the field of genetic engineering, can solve problems such as shortening the process flow, achieve large application space, improve efficiency, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, mutation of chitinase SsChi18A and construction of recombinant vector
[0024] (1) Obtain chitinase SsChi18A gene from NCBI database, connect SsChi18A gene and plasmid pET28a to obtain pET28a-SsChi18A plasmid, the nucleotide sequence of the pET28a-SsChi18A plasmid is shown in SEQ ID No.3. Using the above-mentioned recombinant plasmid as a template, design mutation primers for site-directed mutation. The primer sequences are as follows:
[0025] K186A-sense: TCGGACGGAGGCGCACTCGACGCGG;
[0026] K186A-antisense: GAGTGCGCCTCCGTCCGAGGCGTCC;
[0027] The PCR amplification reaction system is as follows:
[0028]
[0029] The reaction procedure is as follows:
[0030] Preheating, 94°C, 5min; denaturation, 94°C, 30s; annealing, 55-65°C, 30s; 30cycles; extension, 72°C, 2min; re-extension, 72°C, 10min; 4°C, forever.
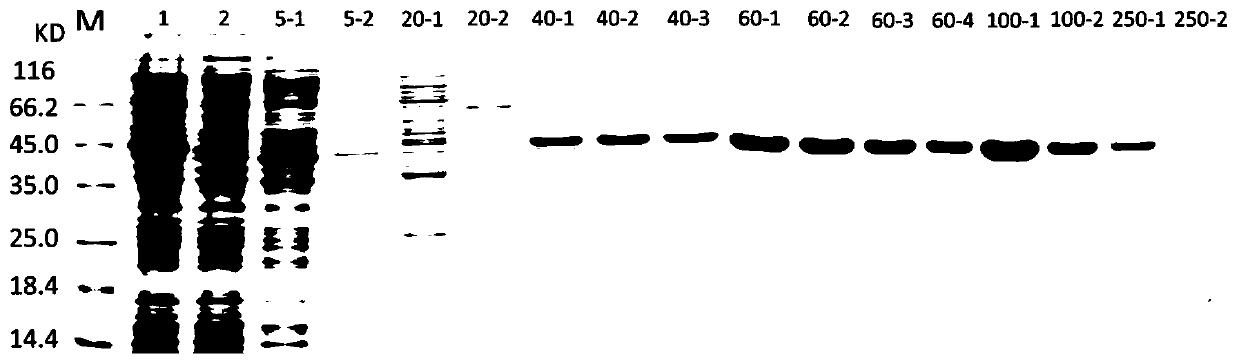
[0031] (2) Take 3 μL of the PCR product for agarose gel electrophoresis verification, leaving a reaction product with clear bands. The wild-typ...
Embodiment 2
[0039] Embodiment 2, construct the recombinant engineered bacterium that contains above-mentioned mutant gene SsChi18A_1
[0040] In this example, a recombinant engineering bacterium containing the above-mentioned mutant gene SsChi18A_1 was constructed. The specific steps are as follows: add 2 μL of the above-mentioned mutant plasmid pET28a-SsChi18A-K186A with correct sequencing to 50 μL of Escherichia coli BL-21 competent cells, bathe in ice for 30 minutes; heat at 42°C Hit for 90s; ice bath for 2min, add 1mL liquid LB, incubate in a shaker at 37°C for 1-1.5h; centrifuge at 8000rpm for 2min, discard the supernatant (leave a little bottom liquid). Spread the remaining solution on an LB plate containing 50 μg / mL kanamycin, spread evenly until dry, and incubate overnight at 37°C; pick a single clone the next day, and inoculate it in 5 mL of LB medium containing antibiotics, at 37°C Cultivate overnight at 200 rpm to obtain recombinant engineered bacteria containing the mutant gen...
Embodiment 3
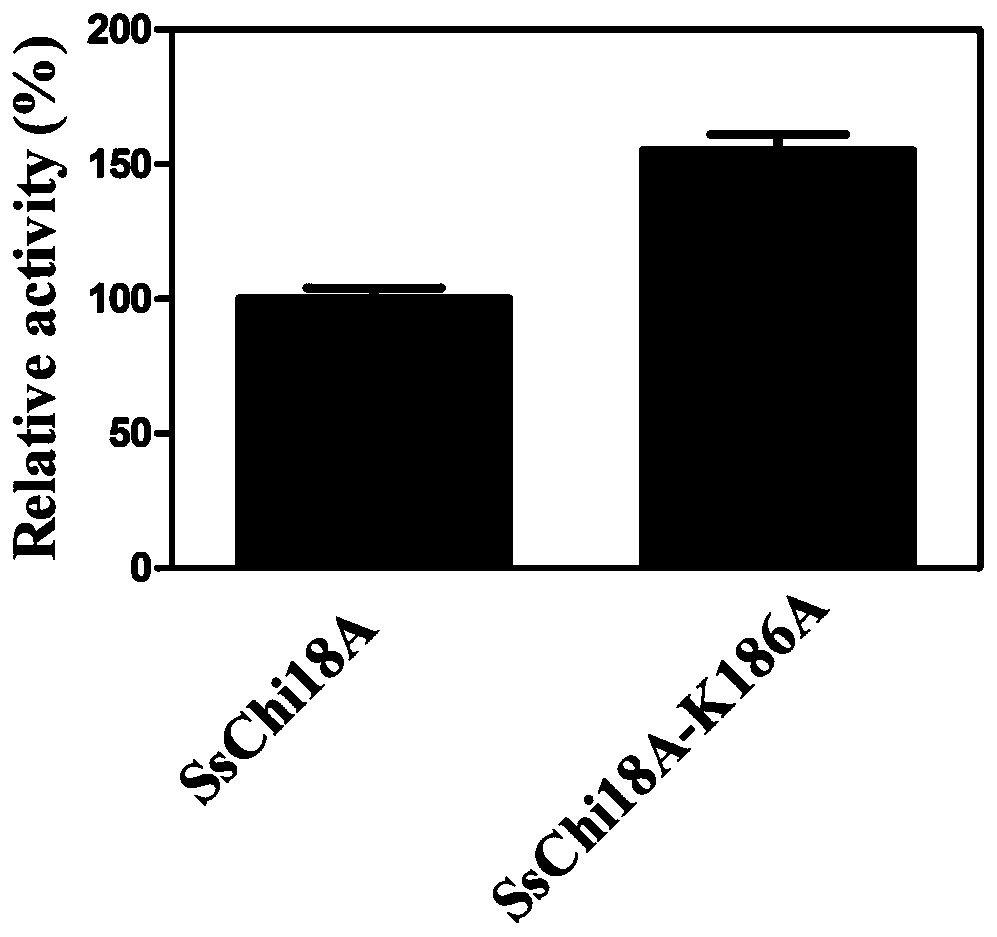
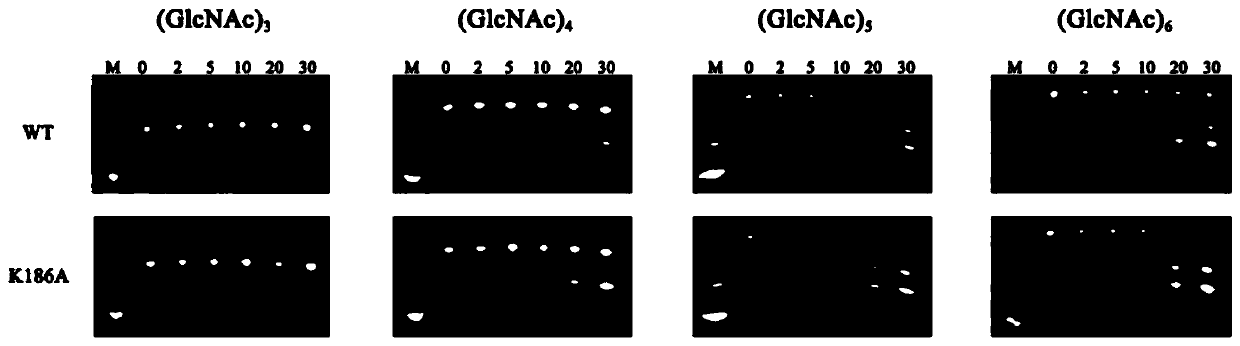
[0041] Embodiment 3: Recombinant expression of mutant gene SsChi18A_1
[0042] Fermentative expression and purification of the mutant chitinase SsChi18A-K186A encoded by the above mutant gene SsChi18A_1, the specific steps are as follows:
[0043] (1) Heterologous expression of recombinant protein:
[0044] Get the recombinant engineering bacterium containing the above-mentioned mutant gene SsChi18A_1 obtained in Example 2, and culture overnight at 37° C. 200 rpm in 5 mL LB medium (containing 50 μg / mL kanamycin);
[0045] Transfer the cultured bacterial solution overnight to a 1L Erlenmeyer flask containing 300mL LB medium (containing 50μg / mL kanamycin), and culture it at 37°C for about 3 hours until OD600=0.6-0.8; the final concentration of adding 0.5 mM IPTG, induced culture at 20°C for 20h; centrifuge at 8000rpm, 4°C for 10min to obtain bacterial pellet;
[0046] NaH at pH 8.0 2 PO 4 -Resuspend the bacteria in NaCl buffer, put the bacteria in a 100mL centrifuge tube; pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com