New application of (R)-omega-transaminase mutant
A technology of mutants and transaminases, applied in the field of molecular biology, can solve the problems of low optical purity, environmental pollution, and low yield, and achieve the effects of strong stereoselectivity, simple preparation process, and easy separation and extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Experimental materials
[0028] (1) LB medium: 10g / L tryptone (purchased from Oxoid), 5g / L yeast powder (purchased from Oxoid), 10g / L sodium chloride (purchased from Sangon Bioengineering Co., Ltd. (Shanghai)), pH 7.0. LB solid medium: add 2% (mass ratio) agar powder to LB liquid medium.
[0029] (2) 2,3-dichloroacetophenone, 2,5-dichloroacetophenone and Marfey reagent were purchased from Aladdin Reagent Co., Ltd. Sodium chloride, glycerin, calcium chloride, imidazole, glacial acetic acid, disodium hydrogen phosphate, sodium dihydrogen phosphate, pyridoxal 5-phosphate, Coomassie brilliant blue protein concentration determination kit, Ni-NTA chromatography medium, isopropyl Base-β-D-thiogalactoside (IPTG), kanamycin sulfate, coenzyme pyridoxal-5-phosphate (PLP) DNA and protein markers were purchased from Sangon Bioengineering Co., Ltd. (Shanghai) .
[0030] (3) Bacterial strain: ω-transaminase gene (ω-TA gene) (SEQ ID NO.2) General Biosystems (Anhui) Co., Ltd. wa...
Embodiment 2
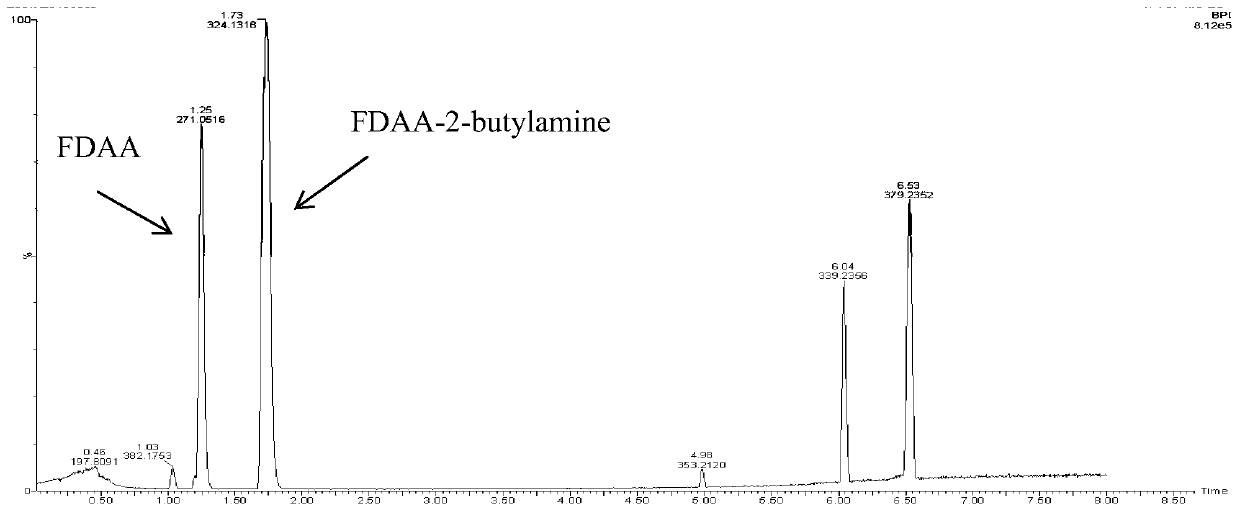
[0069] (R)-ω-transaminase mutants catalyze the transfer of amino groups from amino donors to 2,3-dichloroacetophenone or 2,5-dichloroacetophenone: the substrate solution was phosphate buffered saline (50mmol / L, pH 8.0), the 1mL reaction system includes 50mM sec-butylamine, 30mM aminoacceptor ketones (substrate ketones are 2,3-dichloroacetophenone or 2,5-dichloroacetophenone), 0.1 mM PLP, 0.1mg / mL (R)-ω-transaminase mutant, catalyzed reaction at 30°C for 30h, and the blank control was replaced by buffer solution (R)-ω-transaminase mutant.
[0070] Add 2% of the Marfey chiral derivatization reagent to the reacted mixed solution (the Marfey reagent reacts with the amino groups and other groups in the chiral molecules of the reaction product, and derivates to obtain optical isomers containing two or more chiral centers , through HNMR, HPLC and other analytical means, convenient and quick analysis of the optical purity of the derivatized prochiral compound), derivatized at 40°C for...
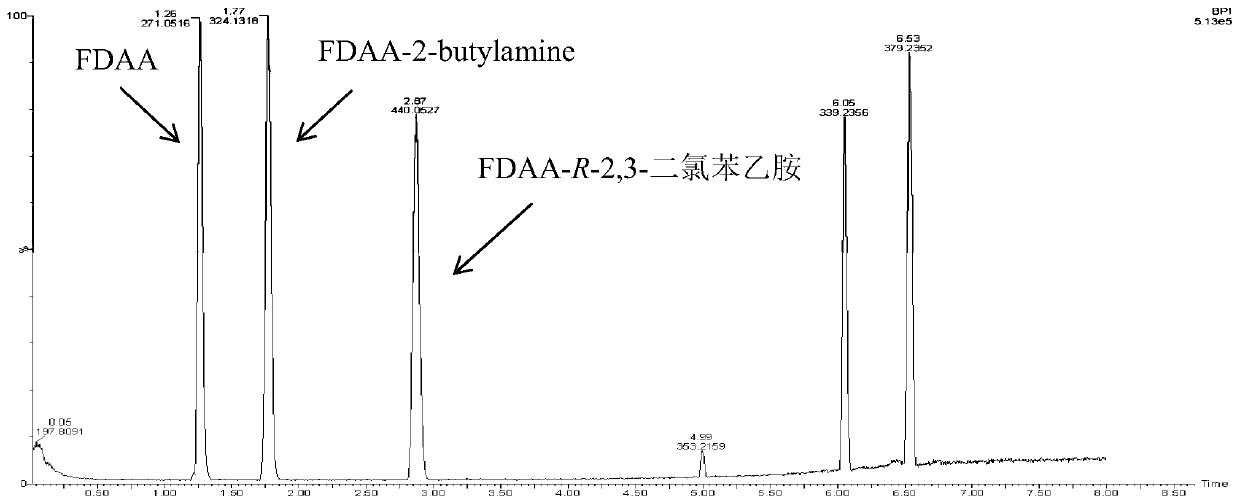
Embodiment 3
[0072] (R)-ω-transaminase mutants catalyze the transfer of amino groups from amino donors to 2,3-dichloroacetophenone or 2,5-dichloroacetophenone: the substrate solution was phosphate buffered saline (50mmol / L, pH 8.0), the 1mL reaction system includes 50mM sec-butylamine, 1mM aminoacceptor ketones (substrate ketones are 2,3-dichloroacetophenone or 2,5-dichloroacetophenone), 0.1 mM PLP, 0.1mg / mL (R)-ω-transaminase mutant, catalyzed reaction at 30°C for 30h, and the blank control was replaced by buffer solution (R)-ω-transaminase mutant.
[0073] The reacted mixture was added with 2% Marfey chiral derivatization reagent, and derivatized at 40° C. for 2 h. After the derivatization was completed, 2M HCl was added to terminate the derivatization. The product was extracted with ethyl acetate, dried by a nitrogen blower, reconstituted in acetonitrile, filtered through a filter membrane, and then filled into a sample bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com