Modified titanium dioxide catalyst with special morphology and its preparation method and application
A titanium dioxide and catalyst technology, applied in the field of titanium dioxide catalyst and its preparation, can solve the problems of limited industrialization and application, dealumination of molecular sieve carrier framework, narrow reaction activity window, etc., and achieves easy operation and implementation, wide activity temperature window, and reaction temperature. Window width effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Synthesis of modified titania catalyst carrier with special morphology
[0063] Take 0.025ml of diethylenetriamine and quickly add it to 40ml of isopropanol, and stir with a magnet at room temperature of 25°C for 20 minutes to obtain a solution of diethylenetriamine in isopropanol. Then, 1.2 ml of titanium tetraisopropoxide was added to the isopropanol solution of diethylenetriamine, and then 0.1 g of niobium pentachloride was added to obtain a mixed solution, which was stirred at room temperature at 25° C. for 20 minutes. After stirring evenly, transfer to a polytetrafluoroethylene hydrothermal reaction kettle, and react at 170°C for 20 hours. After the reaction, the reaction kettle was naturally cooled to room temperature of 25°C, and then the precipitate was centrifuged. The separated precipitate was washed 5 times with absolute ethanol, and then the precipitate was dried at 80°C for 10 hours in a vacuum environment. Finally, the dried precipitate was pla...
Embodiment 2
[0064] Example 2: Preparation of TiO2 Catalyst with Special Morphology Modification
[0065] Take by weighing the modified titania catalyst carrier with special appearance prepared by 1g embodiment 1, meanwhile, take by weighing 0.0445g copper nitrate or ferric nitrate or cerium nitrate (taking copper nitrate as an example here) to be dissolved in dehydrated alcohol, then, Immerse the equal volume of the carrier into the copper nitrate solution, ultrasonically disperse for 20 minutes, and then stir with a magnet for 6 hours. The stirred mixture was dried at 80° C. for 10 hours under vacuum environment. Finally, the dried solid was placed in a quartz tube furnace, and the furnace was continuously fed with air. The temperature of the tube furnace was raised from room temperature 25 °C to 500 °C, and it was stably calcined at the temperature of 500 °C for 3 hours to obtain a crystal with special morphology. Modified titanium dioxide catalyst.
Embodiment 3
[0066] Embodiment 3: the test of titania catalyst reactivity
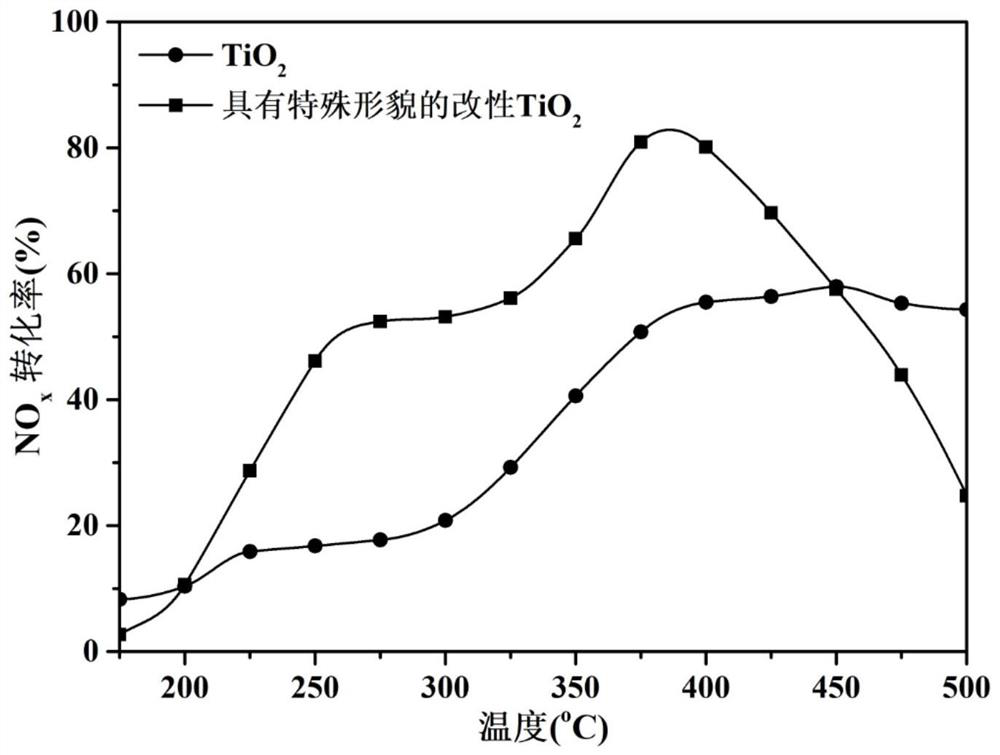
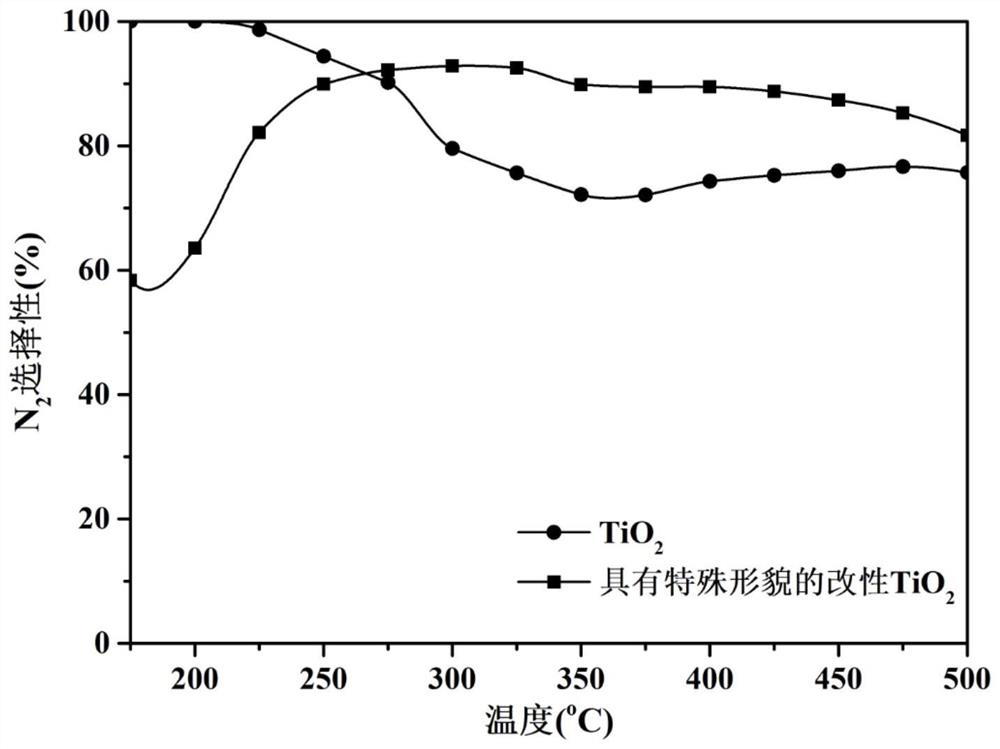
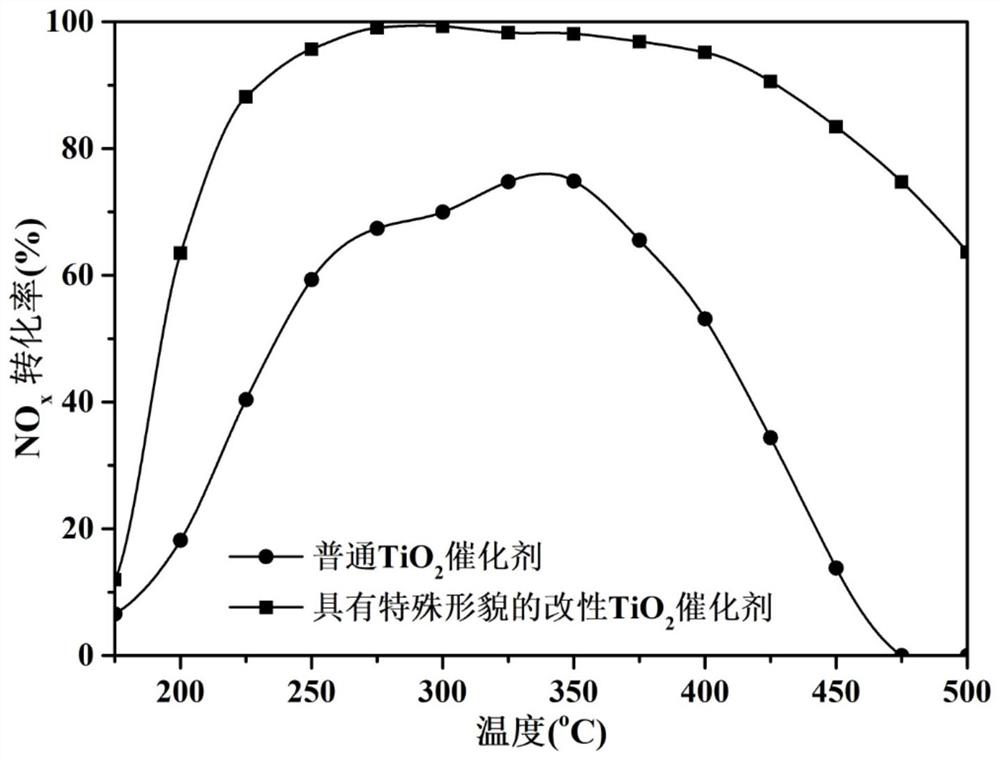
[0067] According to the preparation method of the present invention in Example 1, the dosage of niobium pentachloride was changed to synthesize titanium dioxide carriers with special morphology modified with different contents of niobium element. Several synthesized supports were tested for the catalytic reaction activity of selective catalytic reduction of nitrogen oxides by ammonia according to the following method.
[0068] According to the preparation method of the present invention in Example 2, the amount of nitrate was changed to prepare modified titanium dioxide catalysts with different mass fractions of active components. The prepared catalysts were tested for the catalytic reaction activity of selective catalytic reduction of nitrogen oxides by ammonia according to the following method.
[0069] Sieve the calcined carrier or catalyst into 40-60 mesh granules, take about 0.15g of the sieved catalyst and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com