Method for co-producing sodium bicarbonate and ammonium sulfate from sodium sulfate
A sodium bicarbonate and ammonium bicarbonate technology, applied in carbonate preparations, chemical instruments and methods, ammonium salt fertilizers, etc., can solve problems such as sales difficulties, insufficient product purity, high energy consumption for evaporation and recovery, and achieve significant environmental benefits and economic benefits, avoiding double salt or mixed salt, and eliminating the effect of deamination process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
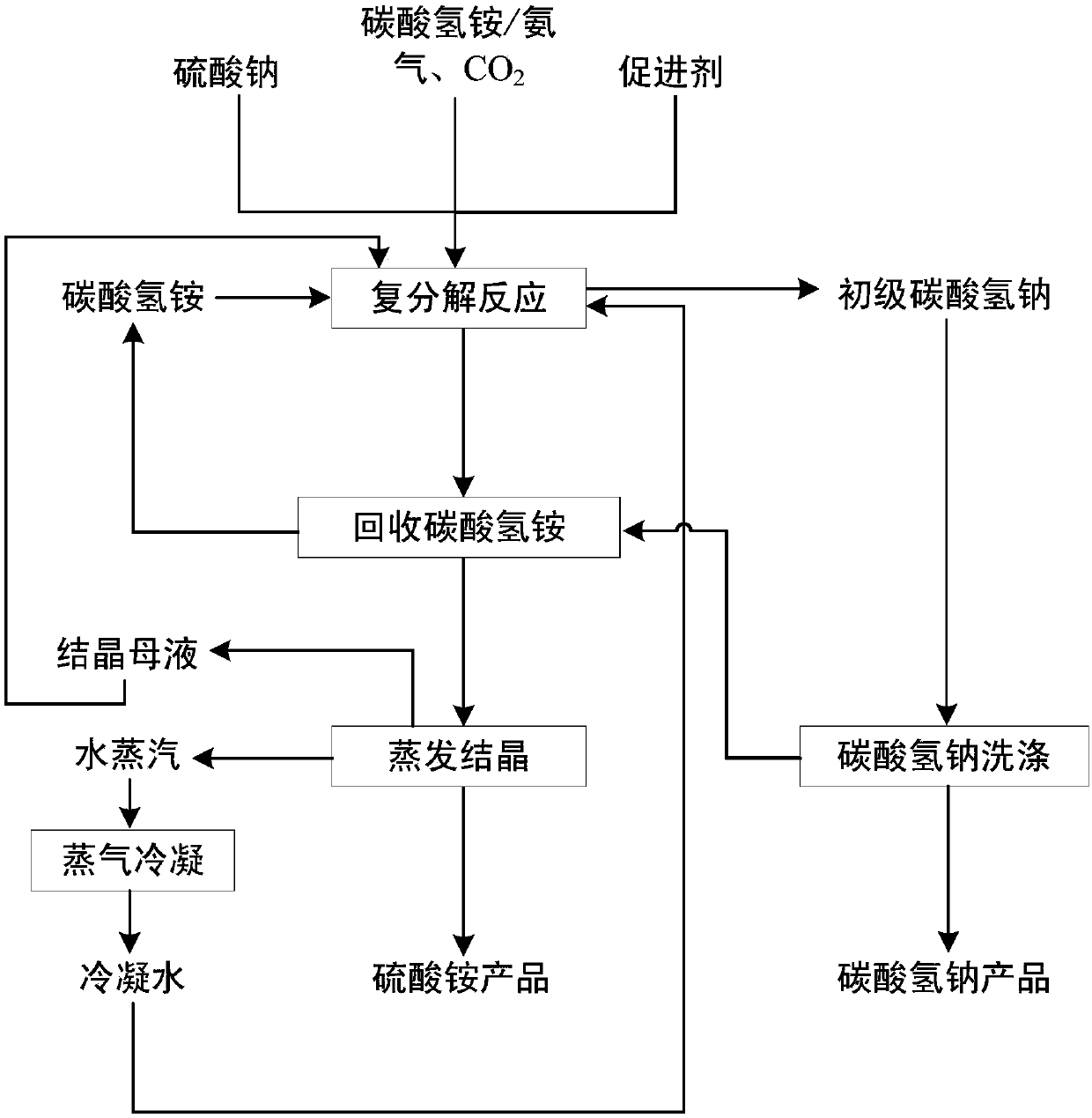
[0050] A kind of method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0051] (1) Mix sodium sulfate, ammonium nitrate, optional condensed water, and crystallization mother liquor, and then slowly add ammonium bicarbonate at 35°C, and ensure the molar ratio of ammonium ions to sodium ions in the solution Not less than 1; after the metathesis reaction is completed, the liquid-solid separation obtains the primary sodium bicarbonate, and the sodium bicarbonate product and the sodium bicarbonate washing liquid are obtained after washing; the addition of ammonium nitrate makes the ammonium nitrate and sulfuric acid in the solution of the metathesis reaction The molar ratio of root ions is 1.5:1;
[0052] (2) Mix the sodium bicarbonate washing solution in the step (1) with the sodium bicarbonate mother liquor obtained from the liquid-solid separation, flash and recycle the ammonium bicarbonate at 80°C, and return the ammonium b...
Embodiment 2
[0056] A kind of method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0057] (1) Mix sodium sulfate, sodium nitrate, optional condensed water, and crystallization mother liquor, and then slowly add ammonium bicarbonate at 35° C., and ensure that the molar ratio of ammonium ions to sodium ions in the solution is not less than 1; After the metathesis reaction is completed, the liquid-solid separation obtains the primary sodium bicarbonate, and the sodium bicarbonate product and the sodium bicarbonate washing liquid are obtained after washing, and the addition of sodium nitrate makes the molar weight of sodium nitrate and sulfate ions The ratio is 2:1;
[0058] (2) The sodium bicarbonate washing solution in step (1) is mixed with the sodium bicarbonate mother liquor obtained by liquid-solid separation, and ammonium bicarbonate is recovered by flash evaporation at 80° C., and the ammonium bicarbonate is returned to step (1);...
Embodiment 3
[0062] A kind of method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0063] (1) Mix sodium sulfate, sodium formate, optional condensed water, and crystallization mother liquor, and then slowly add ammonium bicarbonate at 35°C, and ensure that the molar ratio of ammonium ions to sodium ions in the solution is not less than 1; metathesis After reaction was finished, liquid-solid separation obtained primary sodium bicarbonate, obtained sodium bicarbonate product and sodium bicarbonate washing liquid after washing, the add-on of sodium formate makes the ratio of the molar weight of sodium formate and sulfate ion in the solution that carries out metathesis reaction be 0.7:1;
[0064] (2) The sodium bicarbonate washing solution in step (1) is mixed with the sodium bicarbonate mother liquor obtained by liquid-solid separation, and ammonium bicarbonate is recovered by flash evaporation at 80° C., and the ammonium bicarbonate ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com