Single ion polymer electrolyte and its preparation method and lithium ion battery
A polymer and single-ion technology, applied in the field of lithium-ion batteries, can solve the problems of graft modification without segment, unfavorable industrial scale production, high solution viscosity, etc., to improve service life and safety performance, and avoid mechanical strength reduction , Improve the effect of ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
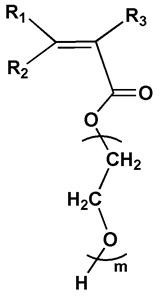
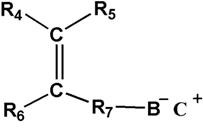
[0096] In this embodiment, the first monomer is polyethylene glycol methacrylate ( The number of repeating units m=19), the second monomer is polyethylene glycol methyl ether methacrylate ( The number of repeating units is y=19), and the third monomer is sodium methylpropanesulfonate.
[0097] Preparation of single-ion polymers
[0098] (1) Preparation of PEGMA
[0099] Under the protection of argon, in a closed reaction vessel, dissolve 10g of the first monomer, 10g of the second monomer and 0.0085g of AIBN in the NMP solvent and mix evenly, then perform at least three degassing operations to ensure that the system is anhydrous and oxygen-free environment, at 70 o C temperature reaction 8h, the end of the reaction. The product was precipitated in hexane, washed, and dried in vacuum for 24 hours to obtain PEGMA with a yield of 92%.
[0100] (2) Preparation of PEGMA-Br
[0101] at 0 o Under the temperature of ℃, the PEGMA of above-mentioned synthesis 15g and the trieth...
Embodiment 2
[0107] In this embodiment, the first monomer is polyethylene glycol methacrylate ( The number of repeating units m=32), the second monomer is polyethylene glycol methyl ether methacrylate ( The number of repeating units is y=28), and the third monomer is 2-acrylamide-2-methylpropanesulfonic acid.
[0108] Preparation of single-ion polymers
[0109] (1) Preparation of PEGMA
[0110] Under the protection of argon, in a closed reaction vessel, dissolve 14g of the first monomer, 6g of the second monomer and 0.0046g of AIBN in the NMP solvent and mix evenly, then perform at least three degassing operations to ensure that the system is anhydrous and oxygen-free environment, at 70 o C temperature reaction 8h, the end of the reaction. The product was precipitated in hexane, washed, and dried in vacuum for 24 hours to obtain PEGMA, weight average molecular weight.
[0111] (2) Preparation of PEGMA-Br
[0112] at 0 o Under the temperature of C, the PEGMA of 15g synthesized abov...
Embodiment 3
[0118] In this embodiment, the first monomer is polyethylene glycol methacrylate ( The number of repeating units m=9), the second monomer is polyethylene glycol methyl ether methacrylate ( The number of repeating units is y=9), and the third monomer is sodium styrene sulfonate.
[0119] Preparation of single-ion polymers
[0120] (1) Preparation of PEGMA
[0121] Under argon protection, in a closed reaction vessel, after dissolving 6g of the first monomer, 14g of the second monomer and 0.0317g of AIBN in a dioxane solvent and mixing them uniformly, perform at least three degassing operations to ensure that the system is free of In an oxygen-free water environment, react at a temperature of 70° C. for 8 hours to end the reaction. The product was precipitated in hexane, washed, and dried in vacuum for 24 hours to obtain PEGMA with a yield of 89%.
[0122] (2) Preparation of PEGMA-Br
[0123] At a temperature of 0°C, 15 g of PEGMA synthesized above was reacted with 0.0037...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com