Organic hole transport material, preparation method and applications thereof
A hole-transporting material and organic technology, used in organic chemistry, semiconductor/solid-state device manufacturing, electrical components, etc., and can solve problems such as poor film-forming performance, poor hole-transporting ability, and low glass transition temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In another typical embodiment, the present invention provides a method for preparing an organic hole transport material. The preparation method includes: S1. First, under the protection of a rare gas (such as nitrogen, argon), weigh a certain The polished magnesium strips of high quality and the distilled first organic solvent are put into the reaction device, then the halogenated thiophene is added dropwise to carry out the first reaction, and finally the Grignard reagent of the halogenated thiophene is obtained; S2, in the rare gas ( Under the protection of such as nitrogen, argon), weigh a certain quality of halogenated triarylamine A and PDCl 2 (PPh 3 )2 Place in the reaction device, add the distilled second organic solvent and add the Grignard reagent of the halogenated thiophene prepared above while stirring to carry out the second reaction to obtain trithiophene aniline B; wherein, the halogenated thiophene is selected from 2- One of chlorothiophene, 2-bromothio...
Embodiment 1
[0061]
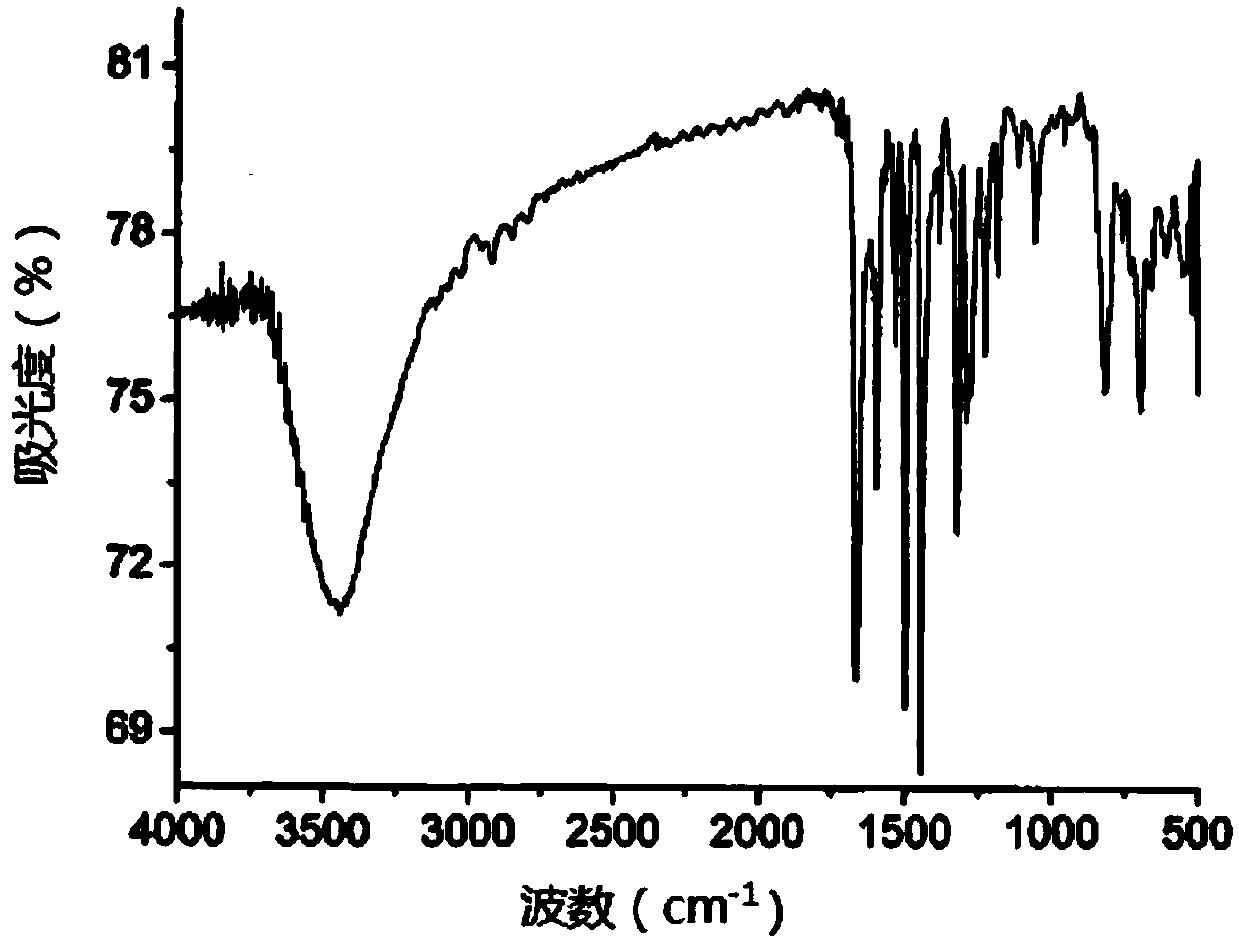
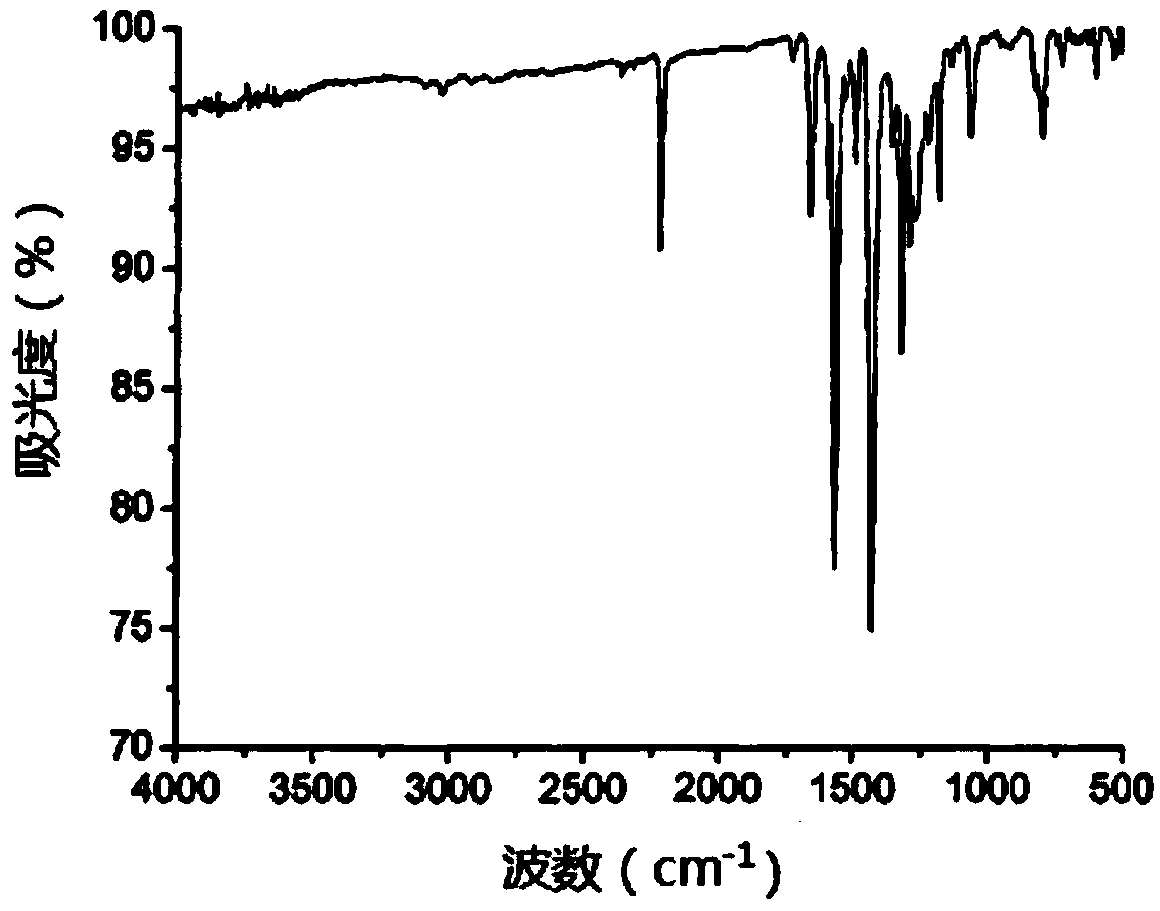
[0062] Weigh 2.528g of polished magnesium strips and place them in a three-necked flask, add 15mL of distilled tetrahydrofuran THF, pass through nitrogen for protection, add 5mL of 2-bromothiophene dropwise with a constant pressure funnel to carry out the first reflux reaction, and stop the reaction after 1h of reflux Grignard reagent for 2-bromothiophene. Then weigh 4.227g tribromotriphenylamine and 0.135g PdCl 2 (PPh 3 ) 2 In a three-necked flask, add 30mL THF and add the Grignard reagent of 2-bromothiophene while stirring, under the protection of nitrogen, heat for the second reflux reaction for 16h, add saturated ammonium chloride to quench the reaction, and then use chloroform The product obtained after quenching is extracted, and the product obtained by extraction is dried with anhydrous sodium acetate, and the eluent composed of n-hexane:dichloromethane with a volume ratio of 3:1 is used for column chromatography on the dried product. Analysis and purific...
Embodiment 2
[0064]
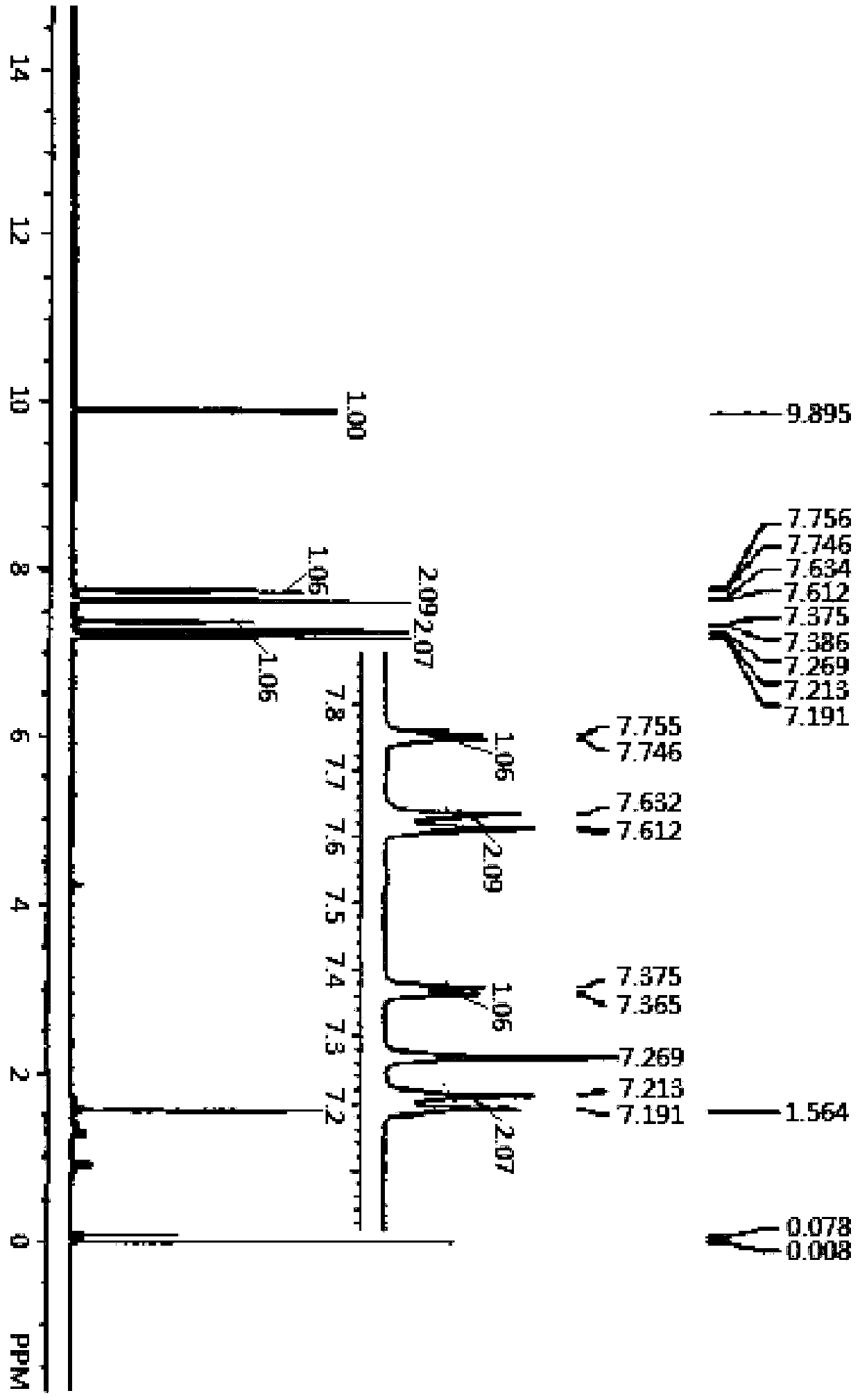
[0065] Weigh 0.613g trithiophene aniline B into a three-necked flask, add 10mL 1,-dichloroethane and 2mL DMF, and stir in an ice bath for 0.5h under the protection of nitrogen, then add 2mL oxychloride dropwise with a constant pressure funnel Phosphorus, heated for the third reflux reaction for 15h, then cooled and poured into ice water, and added 100mL saturated sodium acetate solution to quench the reaction, then extracted the quenched product with 50mL dichloromethane, and simultaneously carried out the organic layer After washing with water for several times, the extracted product was dried with anhydrous sodium sulfate, and finally purified by column chromatography with dichloromethane and ethyl acetate eluent with a volume ratio of 20:1. 0.53 g of product C, tris[4-(5-formyl-2-thienyl)phenyl]amine, was isolated as an orange-yellow solid in a yield of 73.3%. Among them, the infrared spectrum and NMR of tris[4-(5-formyl-2-thienyl)phenyl]amine C 1 H-NMR spectru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com