Preparation method and application of binuclear Ir (III) metal-organic supramolecular cage compound
A supramolecular, organic cage technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve the problems of preparation difficulties, synthesis difficulties, fixed metal coordination methods, etc. Achieve the effect of simple preparation process, stable chemical properties and novel design ideas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Palladium acetate (180mg, 0.8mmol), 4-bromophenylboronic acid (12.0g, 60mmol) and K 2 CO 3 (16.6g, 120mmol) was added to 300mL of a mixed solvent of ethanol and water with a volume ratio of 3:1, and then 4mL of 2-bromopyridine was added to react at 80°C for 1h, cooled to room temperature, extracted with ethyl acetate, anhydrous Dry over sodium sulfate, filter and rotary evaporate, and use petroleum ether and ethyl acetate with a volume ratio of 100:1 to pass through a silica gel column to obtain 6 g of white powder with a yield of 51%. ESI-MS mass spectrum, exact molecular weight 231.988, actual peak 232.988[M+H] + . Mix iridium trichloride (900mg, 3mmol) with white powder (1.77g, 7.7mmol), reflux and stir at 120°C for 10h, after the reaction is completed, filter with suction and wash the obtained filter cake with ethanol, and vacuum dry the washed Filter cake to obtain yellow powder - 1.23 g of dichloro-bridged bromine, with a yield of 62%. ESI-MS mass spectrum, ex...
Embodiment 2
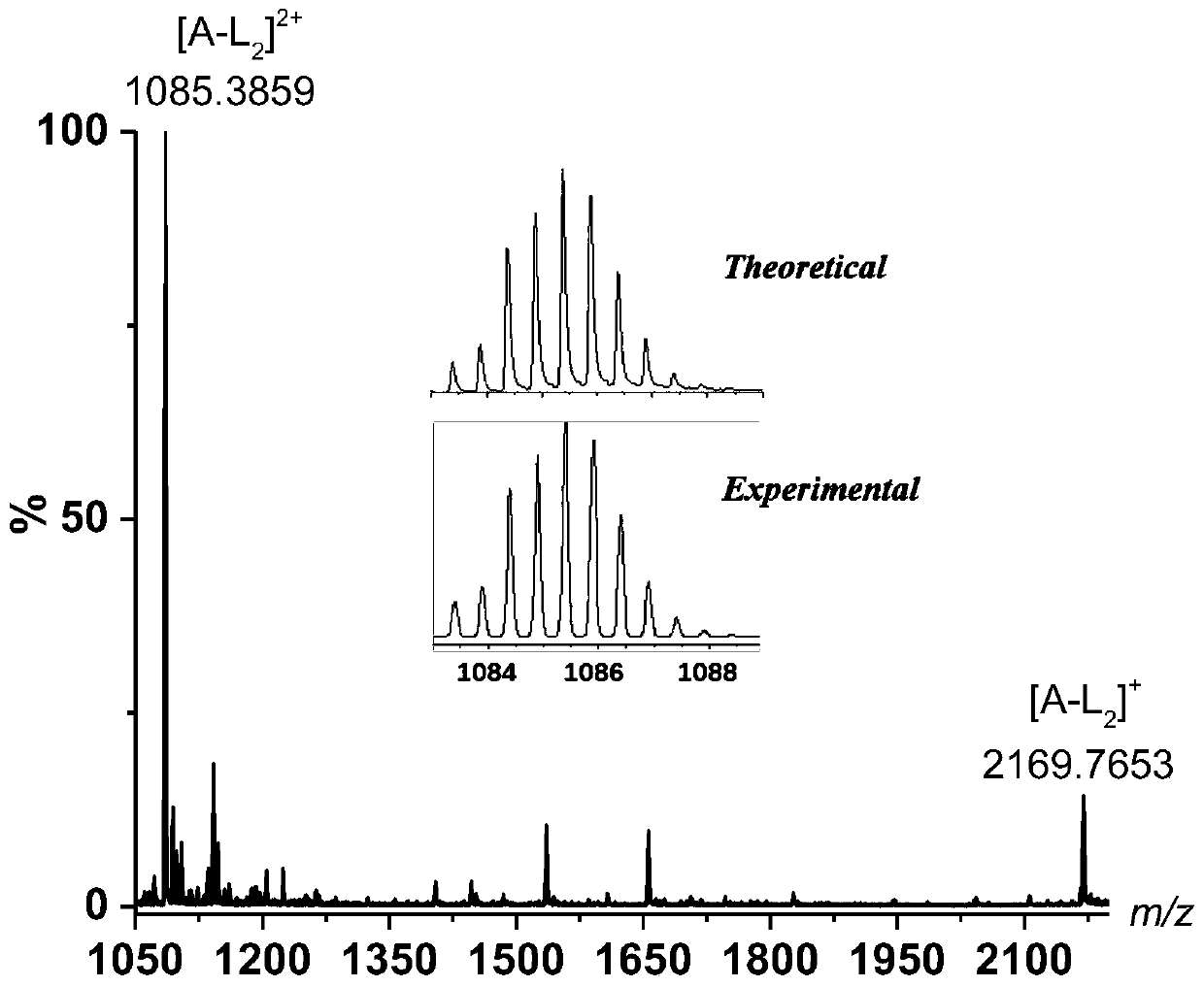
[0036] The pre-assembled metal-based ligand Ir(III) complex L prepared in Example 1 2 (24mg, 0.024mmol) and 1,3 propylenediamine (3mg, 0.0375mmol) were added to a mixed solvent of toluene and acetonitrile with a volume ratio of 2:1, stirred at 110°C for 24h, and then added 1mg of p-toluenesulfonate The acid is used as the catalyst. After the reaction, the solvent is filtered off by suction and washed with acetonitrile for many times to obtain a light yellow powder. Stir the reaction at 0°C for 24 hours, wash with water and extract with dichloromethane several times after the reaction, dry the organic phase with anhydrous sodium sulfate, and evaporate the solvent to obtain the target compound A-L 2 17 mg, yield 64%, ESI-MS mass spectrum: m / z: 1952.319[M+H] + .
Embodiment 3
[0038] The pre-assembled metal-based ligand Ir(III) complex L prepared in Example 1 2 (24mg, 0.024mmol) and 1,2 ethylenediamine ((2.25mg, 0.0375mmol) were added to a mixed solvent of toluene and acetonitrile with a volume ratio of 2:1, stirred at 110°C for 24h, and then 1mg of p- Toluenesulfonic acid is used as a catalyst. After the reaction, the solvent is filtered off by suction and washed with acetonitrile for several times to obtain a light yellow powder. Then the light yellow powder is dissolved in 30 mL of methanol solution, and then 30 mg of sodium borohydride is added in 10 times. Stir the reaction under nitrogen protection at 0°C for 24 hours. After the reaction is completed, wash with dichloromethane and extract several times, dry the organic phase with anhydrous sodium sulfate, and rotary evaporate to dryness to obtain the target compound A-L 2 18mg, yield 68%, ESI-MS mass spectrum: m / z: 1898.6572[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com