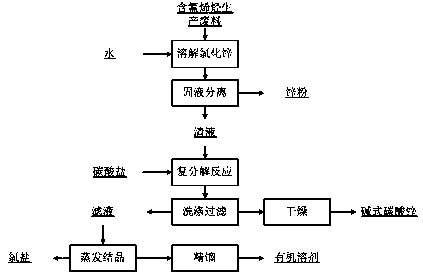
Treatment method of fluorine-containing olefin production waste
A technology for fluorine-containing olefins and waste production, which is applied in the fields of organic chemistry methods, chemical instruments and methods, purification/separation/stabilization of organic compounds, etc. Solvent waste and other problems, to achieve the effect of facilitating industrial scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
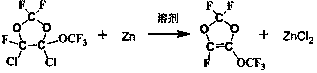
[0035] Add 200 g of 325-mesh zinc powder and 800 g of DMF into a 2L four-necked flask with a mechanical stirrer, a constant-pressure addition funnel, a reflux condensing device, and a thermometer conduit, and add 98.5% of 4,5-dichloro-2,2, Add 500 g of 4-trifluoro-5-trifluoromethoxy-1,3-dioxolane into the constant pressure funnel, start stirring and heating, and start dropping after the internal temperature reaches 80°C. During the dropping process, control The internal temperature is 80-85°C. After the dropwise addition, continue to stir and react at 80°C for 1 hour. The reaction equation is as follows:
[0036]
[0037] The product 2,2,4-trifluoro-5-trifluoromethoxy-1,3-dioxole was collected by an ice-water cold trap. After the reaction was completed, 1135g of the still residue was obtained by DMF, zinc powder and ZnCl 2 composition. The scrap composition is 70.5wt% DMF, 9.3wt% zinc powder and 20.2wt% zinc chloride. Add 250 g of deionized water to the residual liquid of...
Embodiment 2
[0041] Add 300g of water to 1000g of fluorine-containing olefin production waste composed of 55wt% DMF, 12wt% zinc powder and 33wt% zinc chloride, let it stand overnight, recover the lower layer of zinc powder, and obtain 900g of supernatant, add 23% sodium carbonate solution 1550g, ZnCl 2The molar ratio of sodium carbonate to sodium carbonate is 1:1.1, reacted at 60°C, filtered, washed and dried to obtain 287g of basic zinc carbonate. 2523-2016), N.D. in the table means not detected. The filtrate obtained 300g of sodium chloride through evaporation and crystallization, and obtained 512g of DMF with a content of 98.4% after rectification under reduced pressure, and the recovery rate of DMF was 91.6%.
[0042] Table 1 Analytical data of basic zinc carbonate
[0043] Zinc content moisture content Ignition weight loss Manganese (mg / kg) Copper (mg / kg) Cadmium (mg / kg) Lead (mg / kg) Iron (mg / kg) 57.85% 1.48% 25.92% 15.6 1.9 N.D. N.D. 20.0
...
Embodiment 3
[0048] Add 300g of water to 1000g of fluorine-containing olefin production waste composed of 55wt% DMF, 12wt% zinc powder and 33wt% zinc chloride, obtain 102g of zinc powder after centrifugal filtration, add 1420g of 35% potassium carbonate solution to the filtrate, ZnCl 2 The molar ratio with potassium carbonate is 1:1.2, react at 60°C, filter, wash and dry to obtain 280g of basic zinc carbonate. The filtrate was evaporated and crystallized to obtain 491 g of potassium chloride, and the analytical data of potassium chloride are shown in the table below. Obtain 495g of DMF with a content of 99.4% after rectification under reduced pressure, and the DMF recovery rate is 89.5%.
[0049] Table 2 Potassium chloride analysis data
[0050] Calcium (mg / kg) Magnesium (mg / kg) Iron (mg / kg) Potassium chloride (g / 100g) 47.8 3 1.92 98.4
[0051] Using CF 3 OCFCl-CF 2 The reaction between Cl and zinc powder verifies the performance of recovered zinc powder.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com