Fluorine-containing waterborne polyurethane material with waterproof, oil-proof and anti-fouling properties
A water-based polyurethane and anti-fouling technology, applied in anti-fouling/underwater coatings, polyurea/polyurethane coatings, biocide-containing paints, etc., can solve the problems of easy gel effect, increased synthesis process difficulty, and poor reaction controllability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
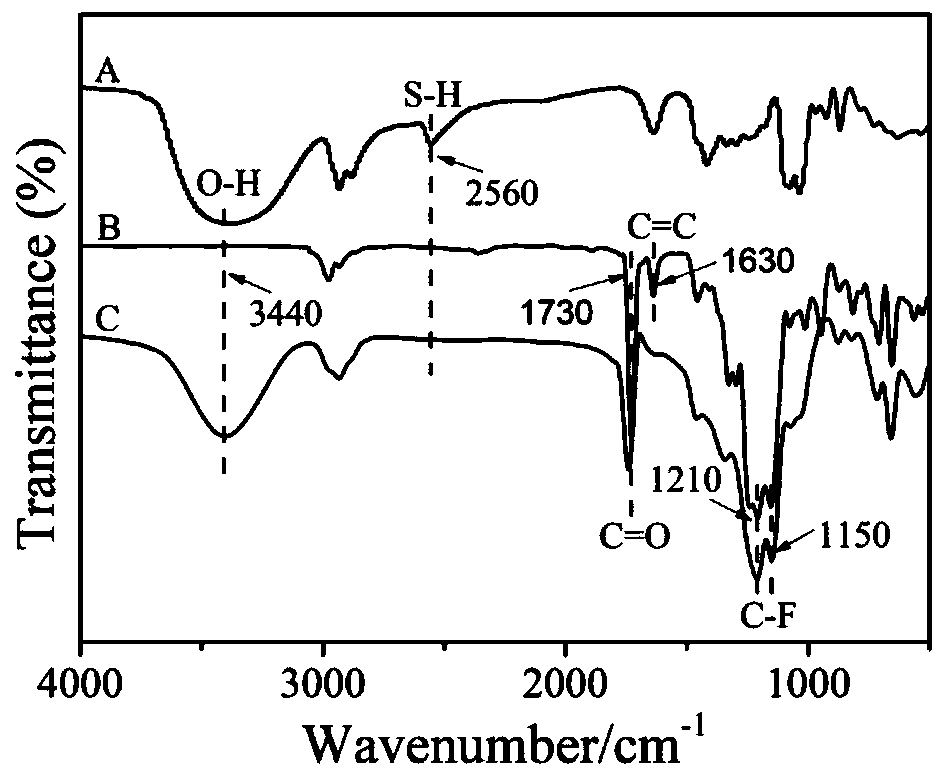
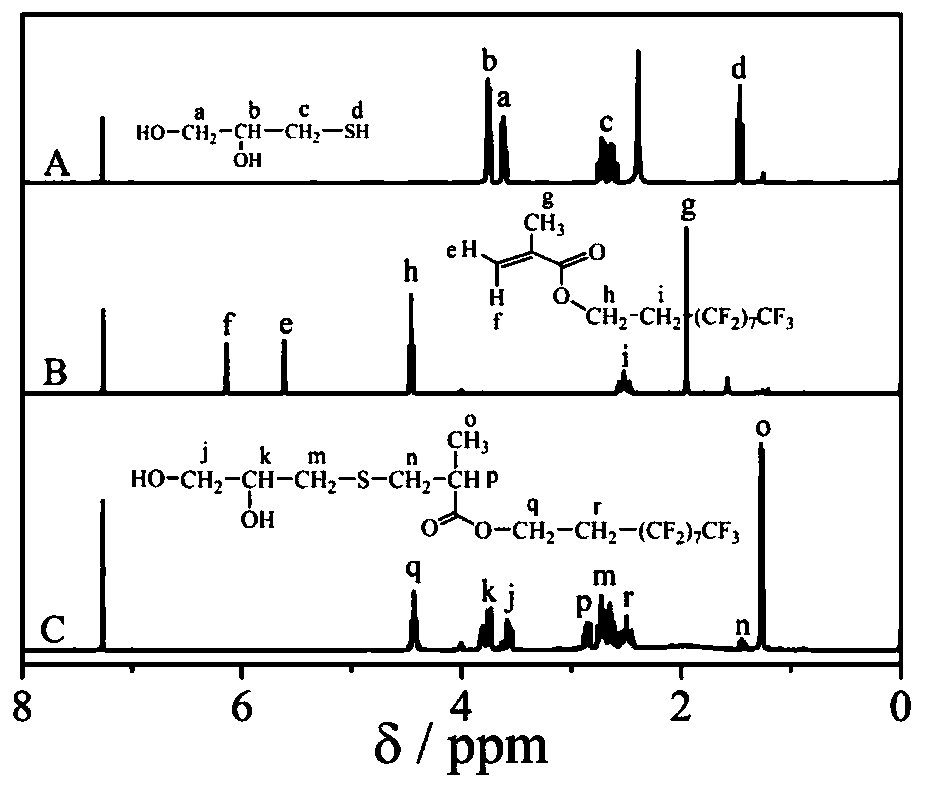
[0029] Example 1: The specific process of the reaction for the preparation of dihydroxyl fluorocarbon chain monomers is as follows: 2.16 g of 3-mercapto 1,2-propanediol, 4.0 g of methacrylic acid 2,2,3,3,4, 4,5,5,6,6,7,7-Dodecafluoroheptyl ester and 0.9 g catalyst triethylamine and 40 mL THF at 35 o C. React for 4 h under nitrogen protection and magnetic stirring. After the reaction was completed, the mixed solution was rotary evaporated to remove most of the solvent, and then the concentrated reaction product was precipitated in water twice for 4 hours each time. After the supernatant was poured off, it was vacuum-dried for 4 hours to obtain the bishydroxyfluorocarbon chain extension Agent monomer A.
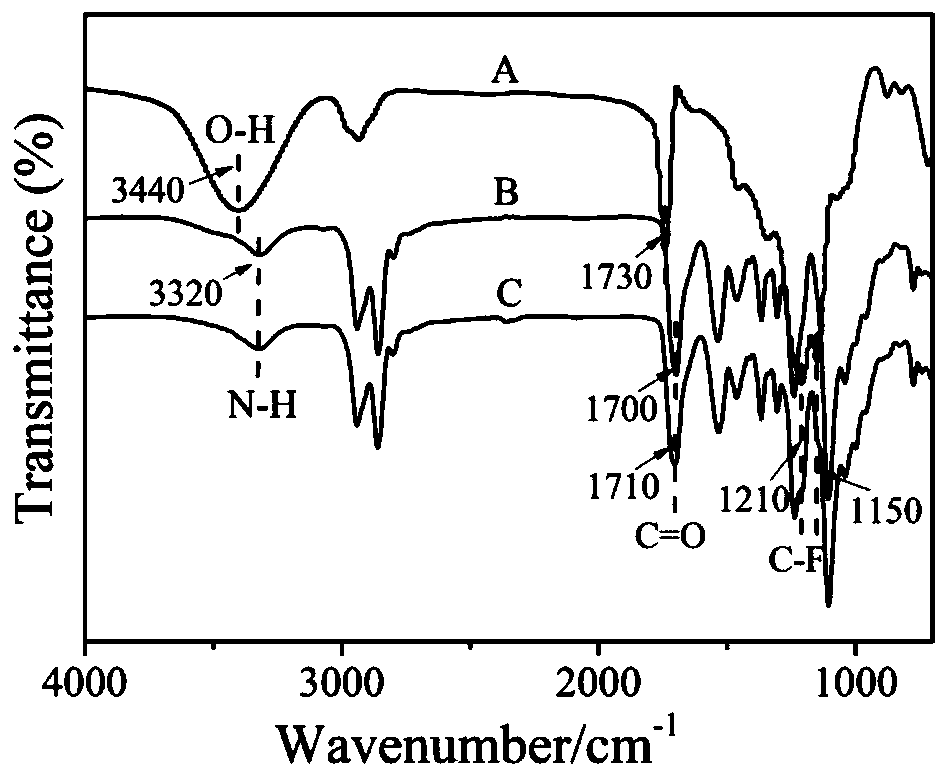
[0030] Weigh 20 g polytetrahydrofuran ether (M n =2000) into a 250 mL three-neck flask, 110 o C vacuum dehydration and drying for 2 h, the temperature dropped to 80 o C, after adding 19.980 g of isophorone diisocyanate and 150 mL of tetrahydrofuran solvent, stir mechanicall...
Embodiment 2
[0031] Example 2: The specific process of the reaction for the preparation of dihydroxyfluorocarbon chain monomers is as follows: add 2.44 g of 4-mercapto 1,2-butanediol and 7.92 g of 2-(perfluorooctyl) ethyl methacrylic acid to a three-necked flask Esters and 1.8 g catalyst triethylamine, 40 mL tetrahydrofuran, at 35 o C. React for 4 h under nitrogen protection and magnetic stirring. After the reaction was completed, the mixed solution was rotary evaporated to remove most of the solvent, and then the concentrated reaction product was precipitated in water twice, 4 hours each time, after the supernatant was poured off, it was vacuum-dried for 4 hours to obtain a bishydroxyfluorocarbon chain extended Agent monomer B.
[0032] Weigh 20 g polycarbonate diol (M n =2000) into a 250 mL three-neck flask, 110 o C vacuum dehydration and drying for 2 h, the temperature dropped to 80 o C, after adding 19.99 g of toluene diisocyanate and 150 mL of tetrahydrofuran solvent, mechanical s...
Embodiment 3
[0033] Example 3: The specific process of the reaction for the preparation of dihydroxyl fluorocarbon chain monomers is as follows: add 2.16 g of 3-mercapto 1,2-propanediol, 6.48 g of methacrylic acid 3,3,4,4,5,5, 6,6,7,7,8,8,8-Tridecafluorooctyl ester and 1.8 g catalyst triethylamine, 40 mL tetrahydrofuran, at 35 o C. React for 4 h under nitrogen protection and magnetic stirring. After the reaction was completed, most of the solvent was removed by rotary evaporation of the mixed solution, and the concentrated reaction product was precipitated in water twice, 4 h each time, and the supernatant was poured off, and then vacuum-dried for 4 h to obtain the bishydroxyfluorocarbon chain extended Chain agent monomer C.
[0034] Weigh 20 g polycaprolactone diol (M n =2000) into a 250 mL three-neck flask, 110 o C vacuum dehydration and drying for 2 h, the temperature dropped to 80 o C, after adding 7.92 g of 1,4-cyclohexane diisocyanate and 150 mL of tetrahydrofuran solvent, mechan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com