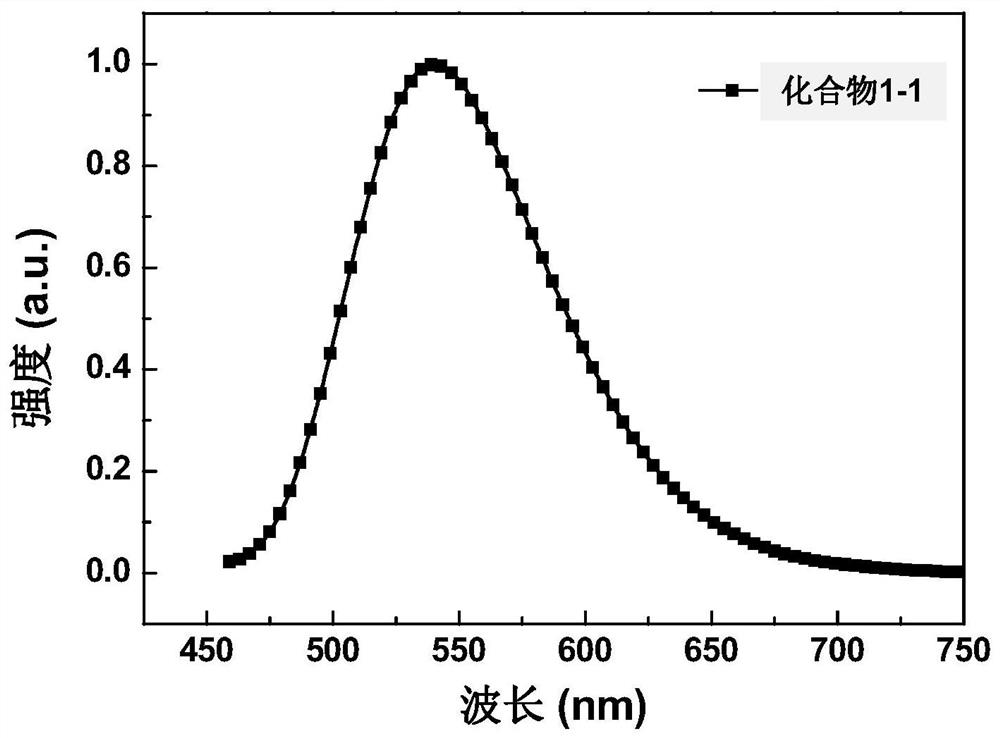
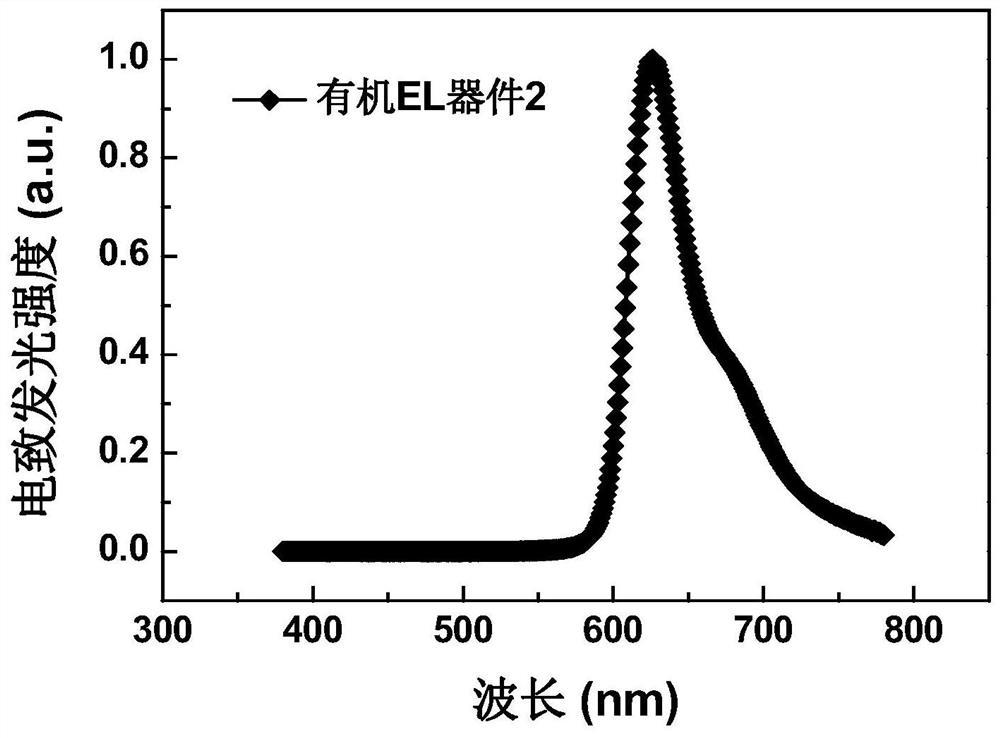
Metatriazine derivative, electronic device and application
A technology of electronic devices and partial triazines, applied in the field of partial triazine derivatives and electronic devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: the synthesis of compound 1-1
[0110] (Synthesis of intermediate M1)
[0111] The synthetic route of intermediate M1 is as follows:
[0112]
[0113] Add p-bromobenzimidic acid hydrazide hydrochloride (5.0g, 20mmol), 4-bromophenylglyoxal hydrate (4.6g, 20mmol) and 120mL absolute ethanol successively in a 250mL single-necked flask, and stir the reaction under reflux 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 5.1 g of a light yellow solid was obtained, with a yield of 65%. MS (EI): m / z: 390.88 [M + ]. Anal.calcdforC 15 h 9 Br 2 N 3 (%): C 46.07, H 2.32, N 10.75; found: C 46.03, H 2.36, N 10.74.
[0114] (Synthesis of compound 1-1)
[0115] The synthetic route of compound 1-1 is as follows:
[...
Embodiment 2
[0118] Embodiment 2: the synthesis of compound 1-17
[0119] (Synthesis of Intermediate M2)
[0120] The synthetic route of intermediate M2 is as follows:
[0121]
[0122] In a 250mL single-necked flask, p-bromobenzimidic acid hydrazide hydrochloride (5.0g, 20mmol), phenylglyoxal hydrate (2.7g, 20mmol) and 120mL absolute ethanol were successively added, and the reaction was stirred under reflux for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 3.7 g of a light yellow solid was obtained, with a yield of 60%. MS(EI): m / z: 312.02[M + ]. Anal.calcd for C 15 h 10 BrN3 (%): C 57.71, H 3.23, N 13.46; found: C 57.69, H 3.25, N 13.43.
[0123] (Synthesis of Compound 1-17)
[0124] The synthetic route of compound 1-17 is as foll...
Embodiment 3
[0127] Embodiment 3: the synthesis of compound 1-58
[0128] (Synthesis of Compound 1-58)
[0129] The synthetic route of compound 1-58 is as follows:
[0130]
[0131] Under nitrogen protection, intermediate M2 (1.6g, 5mmol), bis(4-biphenyl)amine (1.7g, 5.2mmol), palladium acetate (11mg, 0.05mmol), tritert-tert. Butylphosphinetetrafluoroborate (29mg, 0.1mmol), sodium tert-butoxide (960mg, 10mmol) and 120mL toluene were stirred under reflux for 12 hours. After the reaction is complete, evaporate the solvent, dissolve the residue with 200 mL of dichloromethane and 50 mL of water, wash with water, separate the organic layer, extract the aqueous layer twice with 15 mL of dichloromethane, combine the organic layers, evaporate the solvent, and pass the residue through the column Chromatographic separation (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 1.8 g of a yellow solid was obtained with a yield of 65%. MS(EI): m / z: 552.34[M ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com