Alkaline phosphatase-induced gelation and biomimetic mineralization of silk protein solution
A technology of silk protein and biomimetic mineralization, applied in the field of materials, can solve the problems of clinical medical environment mismatch, difficult to degrade, and application limitations, and achieve the effect of promoting natural healing process and promoting adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
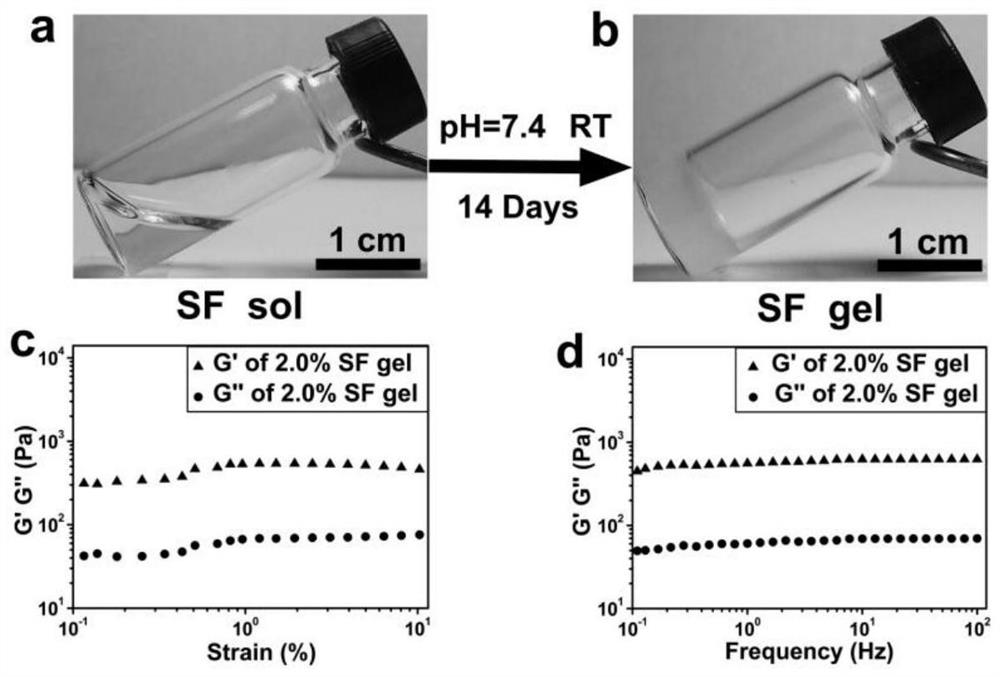
[0036] Embodiment 1: Preparation and purification of silk protein solution
[0037] (1) Silk degumming
[0038] Weigh 4.24g of anhydrous sodium carbonate and dissolve in 2L of boiling deionized water (Na 2 CO 3 Concentration of 0.02M), add 5.0g silk and boil for 1 hour. During the boiling process, the silk should be often stripped to avoid entanglement into bundles. After the end, the silk was pulled out and scrubbed with deionized water for 3-4 times, then air-dried overnight at room temperature, and the weight of the degummed silk was 3.5g, accounting for about 70% of the total weight of the silk.
[0039] (2) silk dissolving
[0040] Weigh 32.3g of anhydrous LiBr to prepare 40mL of a solution with a concentration of 9.3M, and filter with filter paper. 2 g of degummed silk was weighed and added to 12 mL of LiBr solution, and heated and stirred slowly at 60° C. for 4 hours.
[0041] (3) Solution dialysis
[0042] First, wash the dialysis bag with deionized water for 2-3...
Embodiment 2
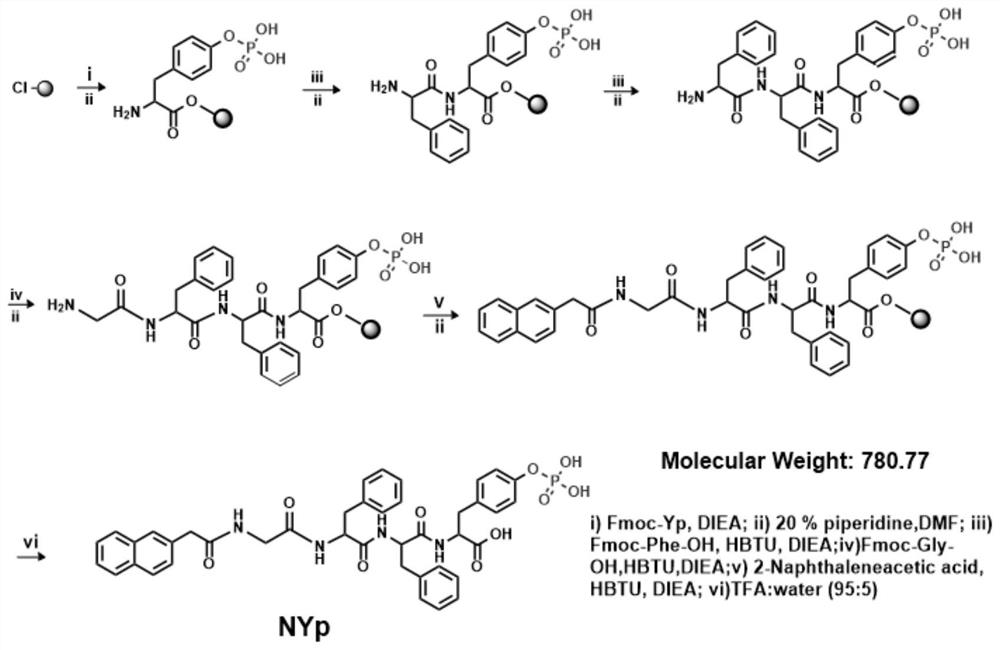
[0053] Example 2: Solid phase synthesis of phosphorylated small molecule polypeptide NYp
[0054] The synthesis process of polypeptide molecule NYp is as follows: figure 1 shown. According to the sequence of the designed target molecule, phosphorylated tyrosine (Fmoc-Tyr(H 2 PO 3 )-OH), phenylalanine (Fmoc-Phe-OH), phenylalanine (Fmoc-Phe-OH), glycine (Fmoc-Gly-OH) and dinaphthylacetic acid (Nap) structural units, specific synthesis Proceed as follows:
[0055] (1) Resin swelling
[0056] Take by weighing 0.5g 2-chlorotrityl resin and join in the solid-phase synthesis reactor, under the effect of nitrogen, add appropriate anhydrous dichloromethane (DCM) swelling resin 30 minutes, extrude anhydrous DCM then and use Washed three times with anhydrous N,N-dimethylformamide (DMF).
[0057] (2) connected to Fmoc-Tyr (H 2 PO 3 )-OH
[0058] Weigh 0.845g Fmoc-Tyr (H 2 PO 3 )-OH was dissolved in 8mL of anhydrous DMF, and 0.76mL of DIEA was added thereto, dissolved with the a...
Embodiment 3
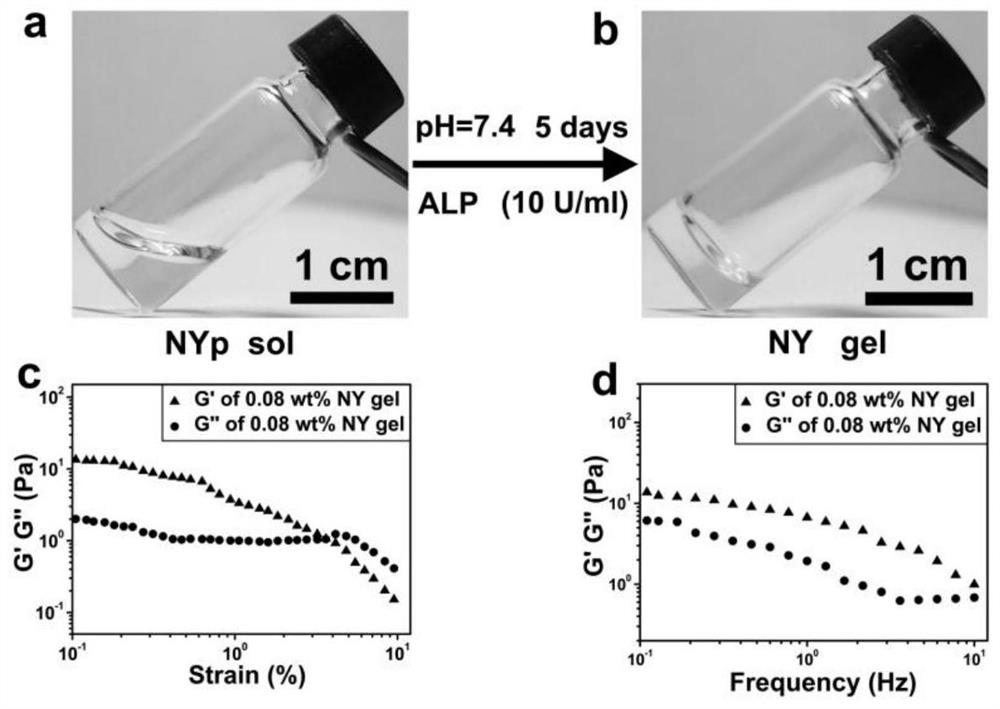
[0084] (1) Weigh 10 mg NYp and dissolve it in a glass vial, adjust the pH by adding an appropriate amount of 1mol / L NaOH to completely dissolve NYp in ultrapure water to form a clear and transparent solution; then add an appropriate amount of 1mol / L HCl to make the system When the pH value is about 7.4, add deionized water to make the total volume 2mL to obtain NYp stock solution with a concentration of 0.5wt%.
[0085] (2) Take a certain amount of silk protein solution with a concentration of 7.9% and add it to a glass vial, adjust the pH value to about 7.4 by adding 1mol / L NaOH, and adjust the volume with ultrapure water to obtain a SF reserve with a concentration of 6.0% and pH=7.4 liquid.
[0086] (4) Pipette 5 μL of SF stock solution into a glass vial, then pipette 30, 48, 60, 120 and 180 μL of NYp stock solution into the SF solution, then add 3 μL of ALP, and finally make the volume to 300 μL. The concentration of SF is 0.1%, and the concentration of NYp is 0.05, 0.08, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com