Drug-loading material and composition thereof of sustained-release preparation, sustained-release preparation and preparation method therefor
A sustained-release preparation and composition technology, applied in the field of medicine, can solve the problems of poor ethanol tolerance, poor tolerance, easy damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
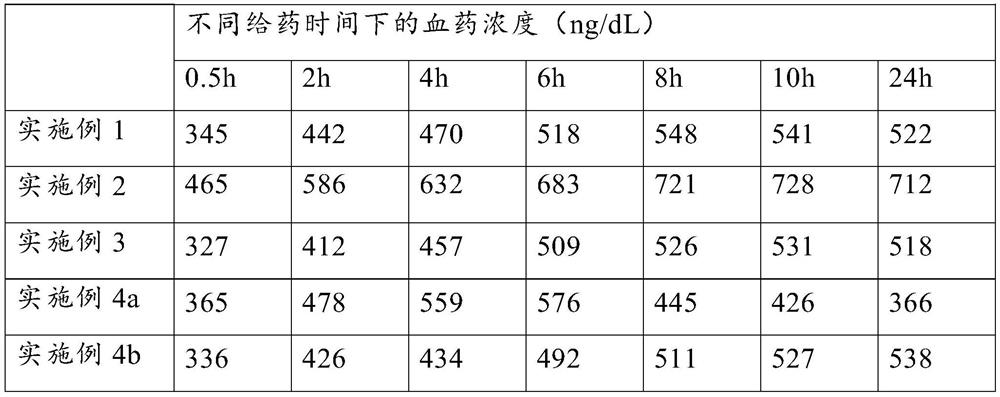
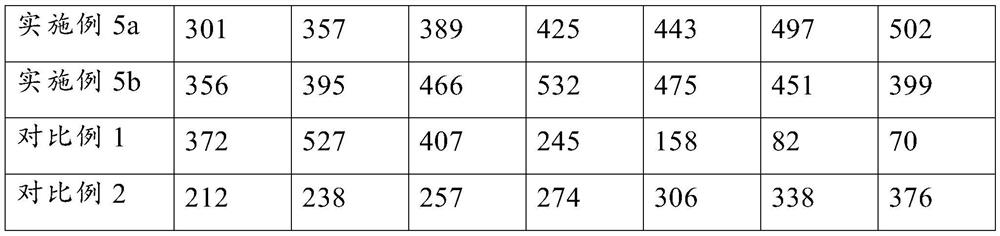
Embodiment 1
[0075] (I) Ingredients
[0076] I1-liposome group:
[0077] Phospholipids: distearoylphosphatidylcholine, 83 parts by weight;
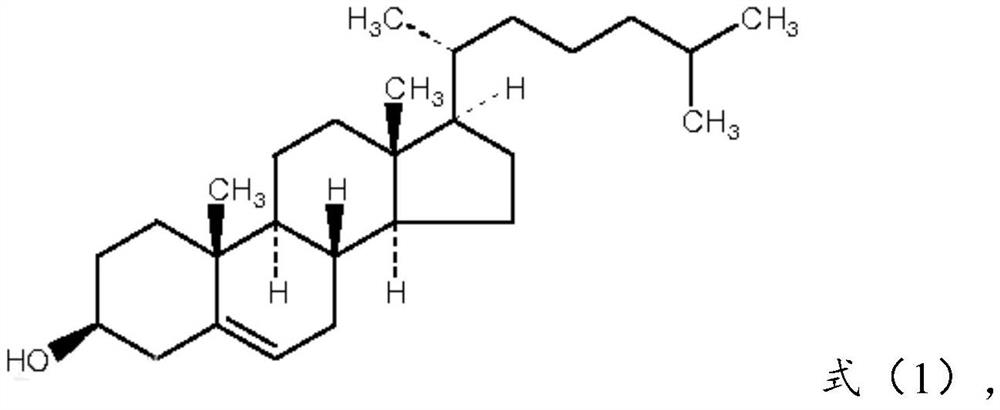
[0078] Cholesterol: compound of formula (1), 17 parts by weight;
[0079] The first penetration enhancer: isopropyl myristate, 187 parts by weight.
[0080] I2-matrix group:
[0081] Aqueous gel compound: hypromellose, 118 parts by weight;
[0082] Fatty acid ester: monoglyceride stearate, 4137 parts by weight;
[0083] The second penetration enhancer: isopropyl myristate, 53 parts by weight.
[0084] I3-Other:
[0085] Medicine: testosterone, 120 parts by weight;
[0086] Solvent: dehydrated alcohol, 500 parts by weight;
[0087] Phosphate buffer: 22200 parts by weight.
[0088] (II) Preparation of anal sustained-release suppositories
[0089] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0090] II2-Preparat...
Embodiment 2
[0094] (I) Ingredients
[0095] I1-liposome group:
[0096] Phospholipids: egg yolk lecithin, 80 parts by weight;
[0097] Cholesterol: compound of formula (1), 20 parts by weight;
[0098] The first penetration enhancer: N-methylpyrrolidone, 116 parts by weight.
[0099] I2-matrix group:
[0100] Aqueous gel compound: hydroxypropyl cellulose, 81 parts by weight;
[0101] Fatty acid ester: propylene glycol stearate, 2025 parts by weight;
[0102] The second penetration enhancer: N-methylpyrrolidone, 50 parts by weight.
[0103] I3-Other:
[0104] Medicine: dihydrotestosterone, 110 parts by weight;
[0105] Solvent: dehydrated alcohol, 500 parts by weight;
[0106] Phosphate buffer: 22200 parts by weight.
[0107] (II) Preparation of anal sustained-release suppositories
[0108] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0109] II...
Embodiment 3
[0113] (I) Ingredients
[0114] I1-liposome group:
[0115] Phospholipids: soybean lecithin, 85 parts by weight;
[0116] Cholesterol: compound of formula (1), 15 parts by weight;
[0117] The first penetration enhancer: isopropyl myristate, 169 parts by weight.
[0118] I2-matrix group:
[0119] Water-based gel compound: Carbomer 940, 86 parts by weight;
[0120] Fatty acid ester: polyethylene glycol stearate, 3889 parts by weight;
[0121] The second penetration enhancer: isopropyl myristate, 56 parts by weight.
[0122] I3-Other:
[0123] Medicine: methyltestosterone, 125 parts by weight;
[0124] Solvent: dehydrated alcohol, 500 parts by weight;
[0125] Phosphate buffer: 22200 parts by weight.
[0126] (II) Preparation of anal sustained-release suppositories
[0127] II1-Preparation of matrix: heating and melting the prepared aqueous gel compound, fatty acid ester and second penetration enhancer in a water bath at 100°C to obtain an oil phase;
[0128] II2-Prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com