Process method for synthesizing ethyl acetate
The technology of an ethyl acetate and a process method is applied in the field of synthesizing ethyl acetate through a transesterification route of methyl acetate raw materials, which can solve the problems of loss of active components, poor stability of heterogeneous catalysts, etc., and achieves low production energy consumption, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
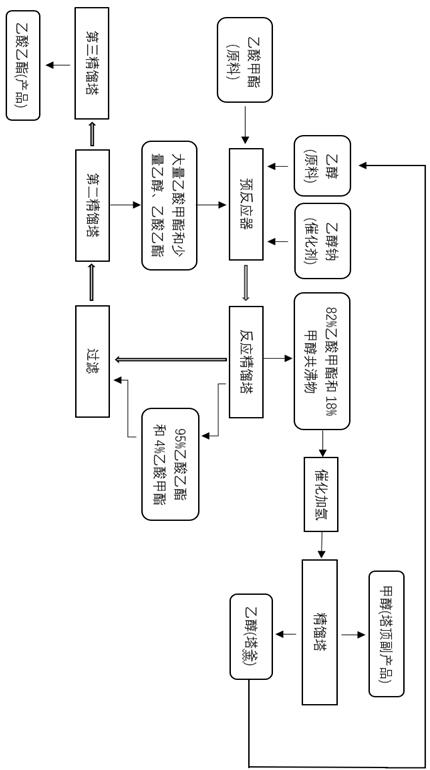
[0039] Such as figure 1 Shown, raw material methyl acetate and ethanol mol ratio are 3.5: 1, react 2 h in the reactor of the homogeneous sodium ethylate catalyst of 1% content after mixing, mixed product is sent to reaction-rectification tower through pumping, in Reaction - In the rectification column, the reaction is continued after separation. The temperature at the top of the rectification tower is 53.8 °C, and the produced components are 82% by mass of methyl acetate and 18% by mass of methanol. Cu-ZnO-Al is used for methyl acetate-methanol azeotrope between 180-250°C 2 o 3 The catalyst undergoes a hydrogenation reaction, and ethanol and methanol are obtained after simple separation. The ethanol is recycled to the reaction raw material, and methanol is a by-product with a purity higher than 99.9%. The reactive distillation column contains ethyl acetate with a content higher than 95%, methyl acetate with a content of about 4%, ethanol with a content of less than 1%, and ...
Embodiment 2
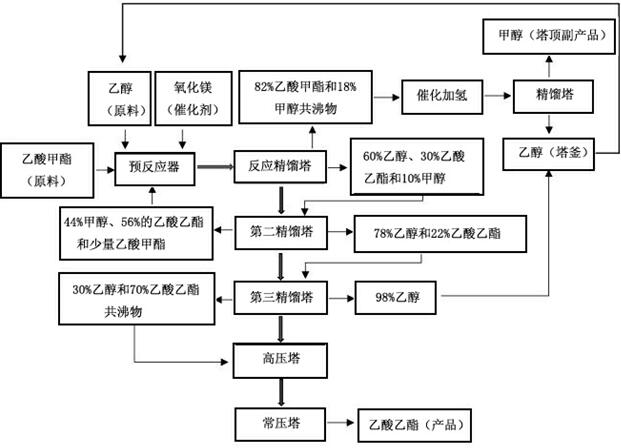
[0041] Such as figure 2 As shown, the raw material methyl acetate and ethanol molar ratio is 1:4, after mixing evenly, pass through the MgO fixed bed pre-reactor prepared by excessive sodium carbonate precipitation method, and the mixed product is pumped to the reaction-rectification tower, and the reaction- The formed solid MgO catalyst is filled in the rectification tower, which is separated in the reaction-rectification tower and then continues to react. The temperature at the top of the rectification tower is 53.8 °C, and the output component is 82% by mass of methyl acetate and 18% of methanol. Cu-ZnO-Al is used for methyl acetate-methanol azeotrope between 180-250°C 2 o 3Catalyst hydrogenation reaction, ethanol and methanol are obtained after rectification and separation, ethanol is recycled to the reaction raw materials, methanol is a by-product, and the purity is higher than 99.9%. At this time, the mass composition of the reaction-rectification tower still is abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com