Organic light-emitting material based on phenanthroimidazole derivatives and application of organic light-emitting material in electroluminescent device
A luminescent material, phenanthroimidazole technology, applied in the field of organic electroluminescence, can solve the problems of chemical instability, high price, large efficiency roll-off, etc., and achieve the effect of high efficiency, low roll-off, and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
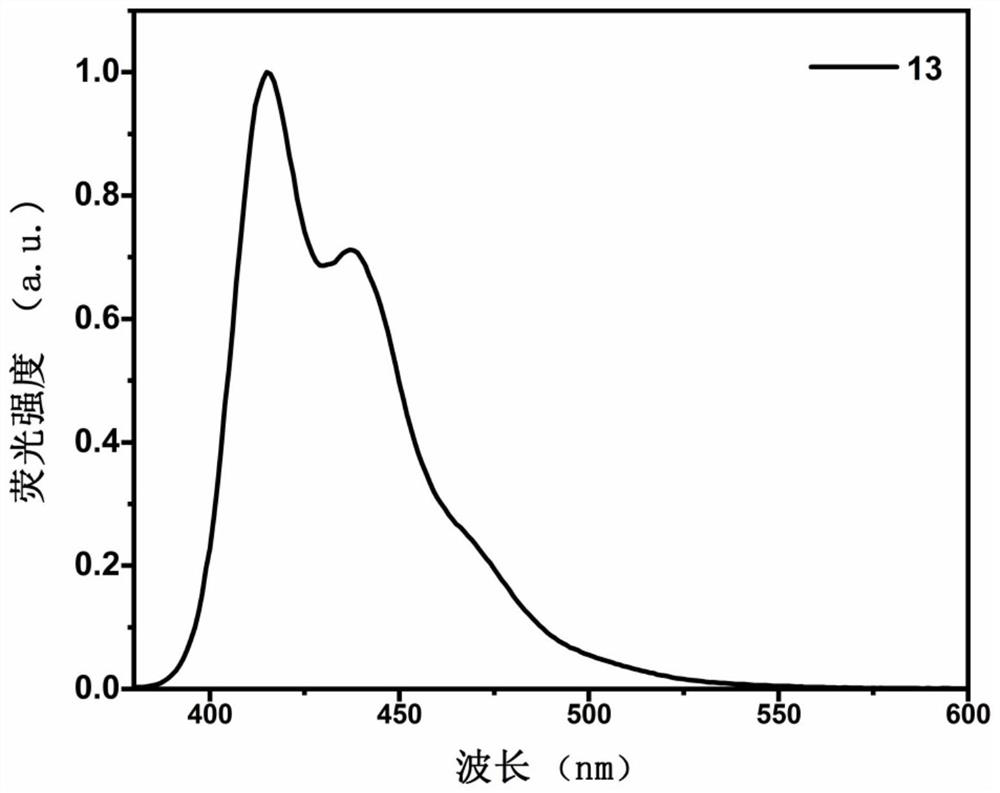
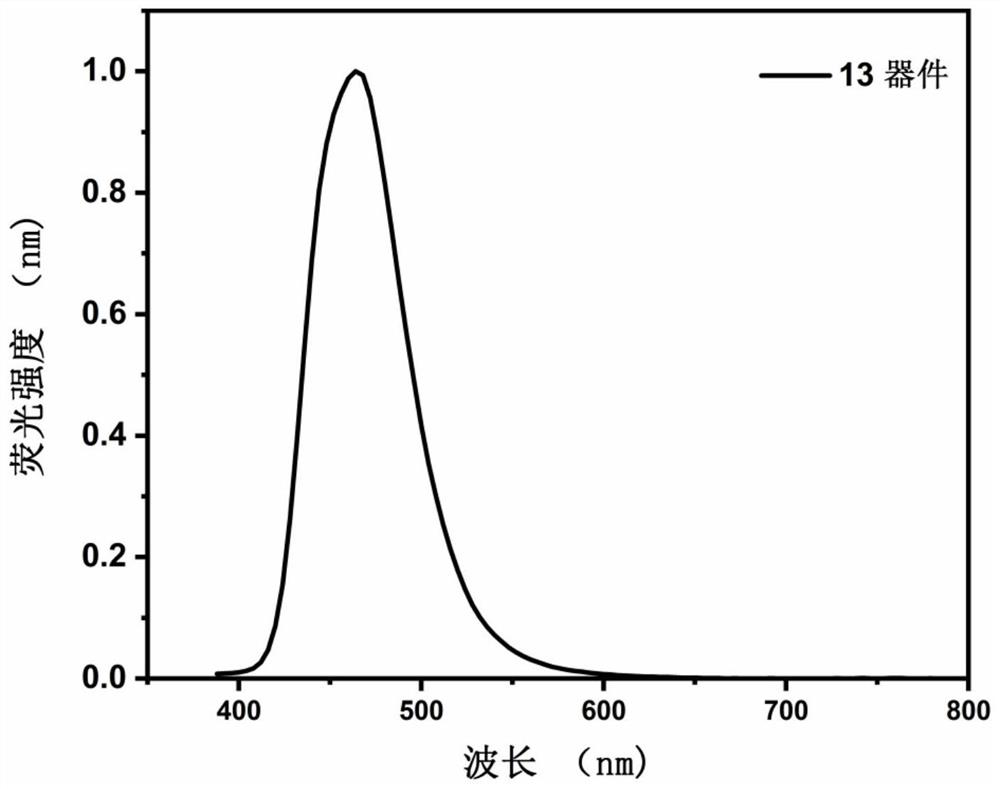
Image
Examples
Embodiment 1
[0060] The concrete preparation method of phenanthroimidazole intermediate is as follows:
[0061]
[0062] 2,7-dibromophenanthrenequinone (10mmol), R 1 -CHO (i.e. R 1 -1 to R 1 -10)(10mmol), R 2 -NH 2 (i.e. R 2 -1 to R 2 -25) (50mmol), ammonium acetate (40mmol), and acetic acid (50mL) were added into a three-necked flask, and heated to reflux in an oil bath at 130°C for 12h under nitrogen protection. The reaction was stopped, the reaction mixture was poured into distilled water, stirred and filtered, and the obtained gray filter cake was washed with water, glacial acetic acid and ethanol in sequence, and dried to obtain the p-bromo-substituted phenanthroimidazole intermediate.
[0063] The specific preparation method of carbon-carbon coupling compound is as follows
[0064] The phenanthroimidazole intermediate (3mmol) substituted by para-bromine, organoboronic acid (i.e. R 3 -1 to R 3 -58) (7mmol), potassium carbonate (24mmol), tetrakis (triphenylphosphine) pallad...
Embodiment 2~27
[0069] As in Example 1, different raw materials were used to prepare compounds 2-27.
Embodiment 28
[0071] The preparation of phenanthroimidazole intermediate is as in Example 1.
[0072] The concrete preparation method of carbon-nitrogen coupling compound is as follows
[0073] The phenanthroimidazole intermediate (3mmol) substituted by para bromine, aromatic amine (ie R 4 -1 to R 4 -21) (7mmol), cesium carbonate (24mmol), three (dibenzylideneacetone) dipalladium (0.1mmol), tri-tert-butylphosphine (0.1mmol), o-xylene (30mL) add in the there-necked flask, N 2 Under protection, heat and reflux in an oil bath at 130°C for 12 hours to stop the reaction, pour the reaction mixture into distilled water, extract with dichloromethane, concentrate and separate by column chromatography (silica gel, dichloromethane) to obtain the powdery target product
[0074] Taking compound 28 as an example to illustrate the specific details of the synthesis example: the phenanthrene imidazole intermediate (3 mmol) substituted by para-bromine, R 4 -3 (7mmol), cesium carbonate (24mmol), three (dib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com