N-type organic semiconductor material as well as preparation method and application thereof
A semiconductor and organic technology, applied in the field of organic optoelectronics, can solve the problems of low carrier splitting efficiency and large energy conversion efficiency gap, and achieve the effects of strong electron transport performance, high photoelectric conversion performance, and high absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of Compound 10
[0041]
[0042] (1) Synthesis of compound 1
[0043] Under the protection of nitrogen, add 1-bromo-2-fluoro-4,5-xylene (10mmol) and sodium hydroxide (20mmol) into 50ml potassium permanganate aqueous solution, start stirring and heat to reflux at 100°C, and react for 10 hours . After the reaction, the product was extracted with ethyl acetate, washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, the dried solution was filtered, and the solvent was spin-dried by a rotary evaporator to obtain a crude product. The crude product was separated and purified by silica gel chromatography, and the eluent was a mixed solvent of petroleum ether / ethyl acetate to obtain a solid product with a yield of 90%. 1 H NMR, 13 The results of C NMR, MS and elemental analysis showed that the obtained compound was the target product.
[0044] (2) Synthesis of compound 2
[0045] Under the protection of nitrogen, compou...
Embodiment 2
[0063] Synthesis of Polymers P1~P4
[0064]
[0065]
[0066] (1) Synthesis of Polymer P1
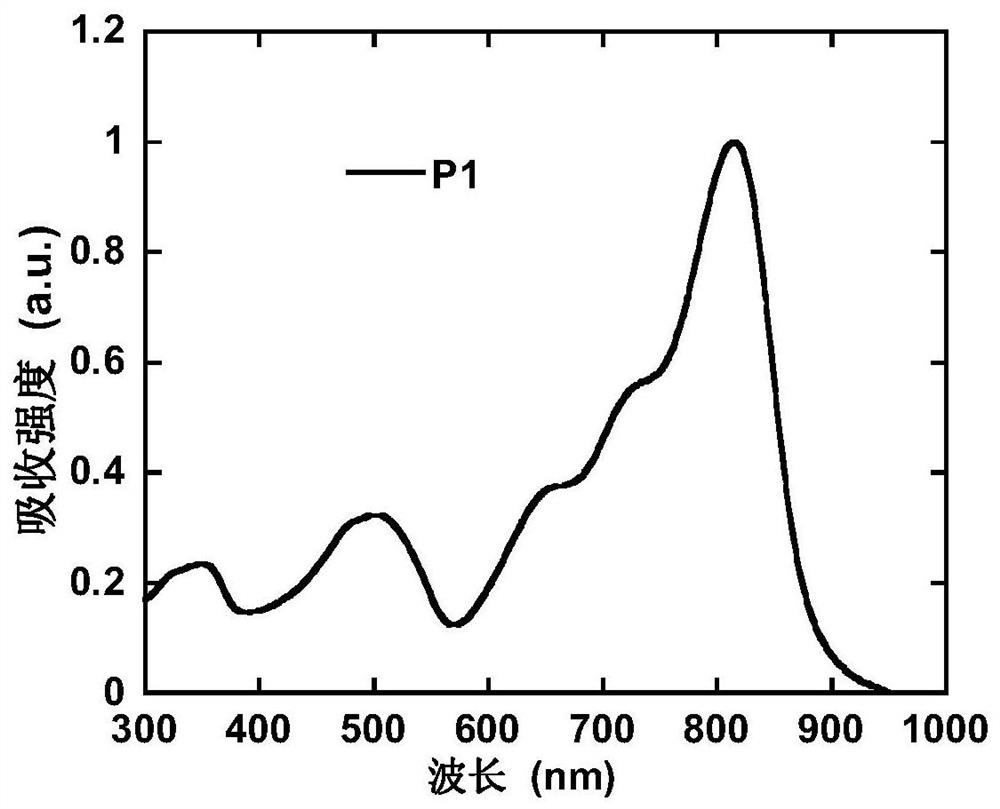
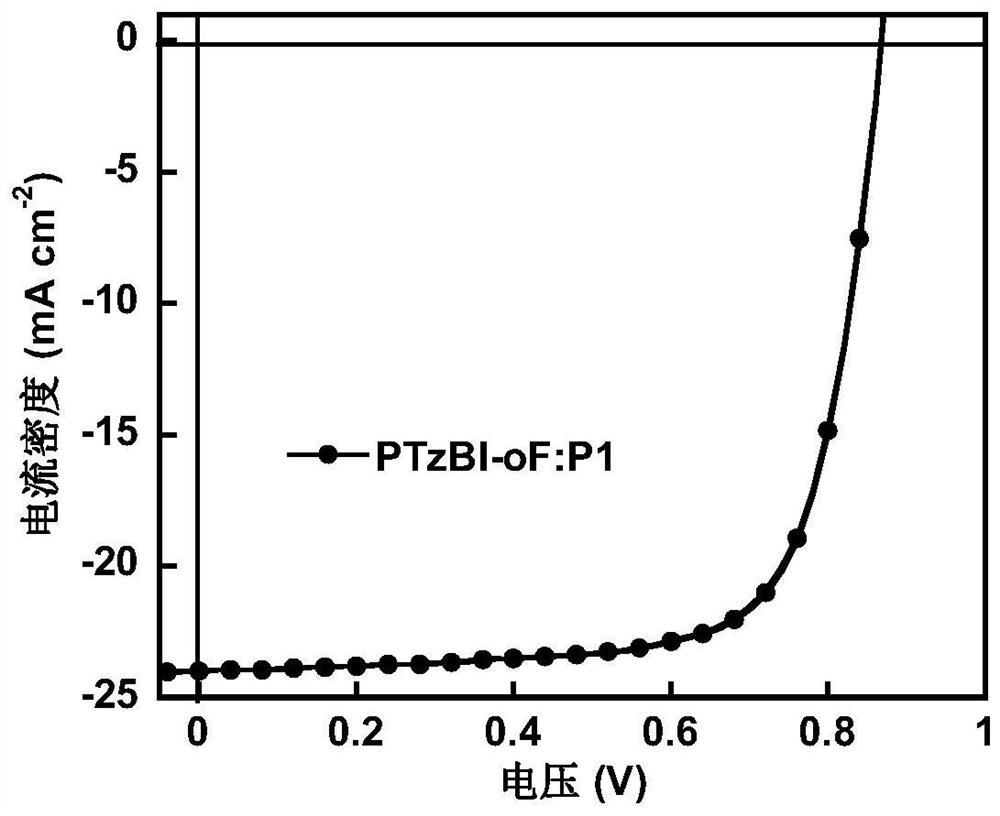
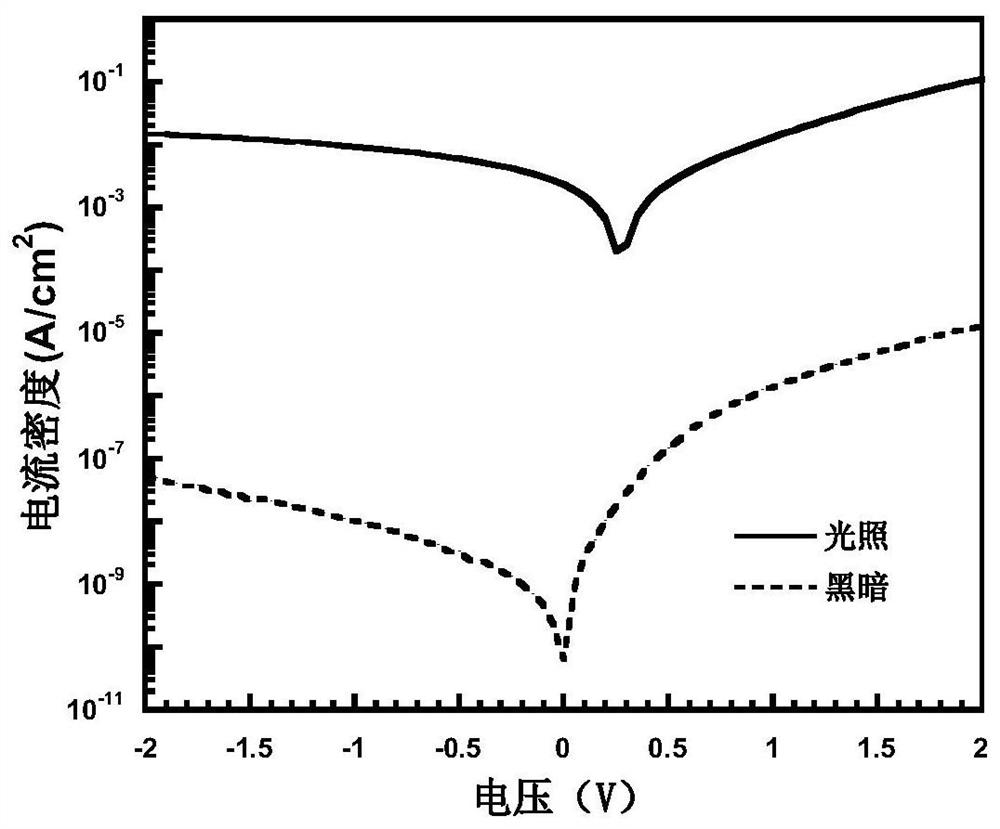
[0067] Under nitrogen protection, compound 10 (1mmol), 2,5-bis(trimethyltin)thiophene (1mmol), tridibenzylideneacetone dipalladium (0.05mmol), tri-tert-butylphosphine (0.1mmol) were dissolved In 10 ml of chlorobenzene, react at 110° C. for 10 hours. After the reaction, the Soxhlet extraction method was used to extract with n-hexane, acetone, dichloromethane, and chloroform solvents in sequence, and finally, the solid product was obtained by chromatography in anhydrous methanol and filtered, with a yield of 90%. 1 H NMR, 13 The results of C NMR, MS and elemental analysis showed that the obtained compound was the target product. figure 1 is the absorption spectrum of polymer P1, and P1 has absorption in a wide wavelength range from 300 to 900 nm.
[0068] (2) Synthesis of Polymer P2
[0069] Under nitrogen protection, compound 10 (1mmol), bis(trimethyltin)bithiophene (1mmol), t...
Embodiment 3
[0075] Synthesis of Polymer P5
[0076]
[0077] (1) Synthesis of Polymer P5
[0078] Under nitrogen protection, compound 10 (1mmol), bis-(1,5-cyclooctadiene) nickel (0.05mmol), and 2-bipyridine (0.5mmol) were dissolved in 10ml of anhydrous tetrahydrofuran, and at 80°C React for 10 hours. After the reaction, the Soxhlet extraction method was used to extract with n-hexane, acetone, dichloromethane, and chloroform in sequence, and finally, the solid product was obtained by chromatography in anhydrous methanol and filtration, with a yield of 89%. 1 H NMR, 13 The results of C NMR, MS and elemental analysis showed that the obtained compound was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com