Polylactic acid-polypeptide micelle and application thereof
A technology of polylactic acid and micelles, which is applied in the field of biomedicine, can solve the problems of changing the structure and function of nucleic acid and drugs, affecting the therapeutic effect and safety, and having no therapeutic effect, so as to promote cell uptake, reasonable particle size and potential , the effect of avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
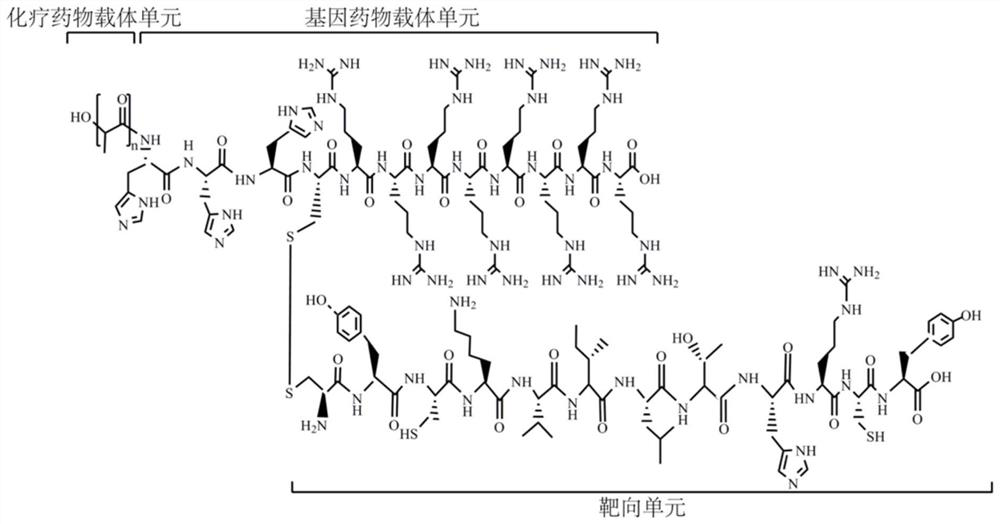
[0043] Example 1 Synthesis of Targeted Co-delivery of Chemotherapeutic Drugs and Gene Drug Carrier PHRD
[0044] ① Protect polylactic acid (PLA) with TBDMS for hydroxyl groups, activate with dicyclohexylcarbodiimide (DCC) and HOBt, remove dicyclohexylurea (DCU) by filtration, add H-His(Trt)-OH to react overnight to obtain TBDMS-polylactic acid-His(Trt)-OH;
[0045] ②Deprotect Fmoc-Arg(Pbf)-Wang Resin with Pip / DMF for 30min, and put in amino acid (AA), tetramethyluronium hexafluorophosphate (HBTU) and N-methylmorpholine (NMM) in an equivalent ratio, Add an appropriate amount of DMF, and react with nitrogen gas for 30 minutes, and the resin sample is transparent when ninhydrin is detected;
[0046] ③ Repeat step ② until the sequence is coupled to the penultimate amino acid;
[0047] ④ Add TBDMS-polylactic acid-His(Trt)-OH, HBTU and NMM to the reactor in proportion, couple for 2 hours, take a sample of the resin and detect it with ninhydrin, wash it with methanol, drain the res...
Embodiment 2
[0050] Example 2 Characterization of targeted co-delivery of chemotherapeutic drugs and gene drug carrier PHRD
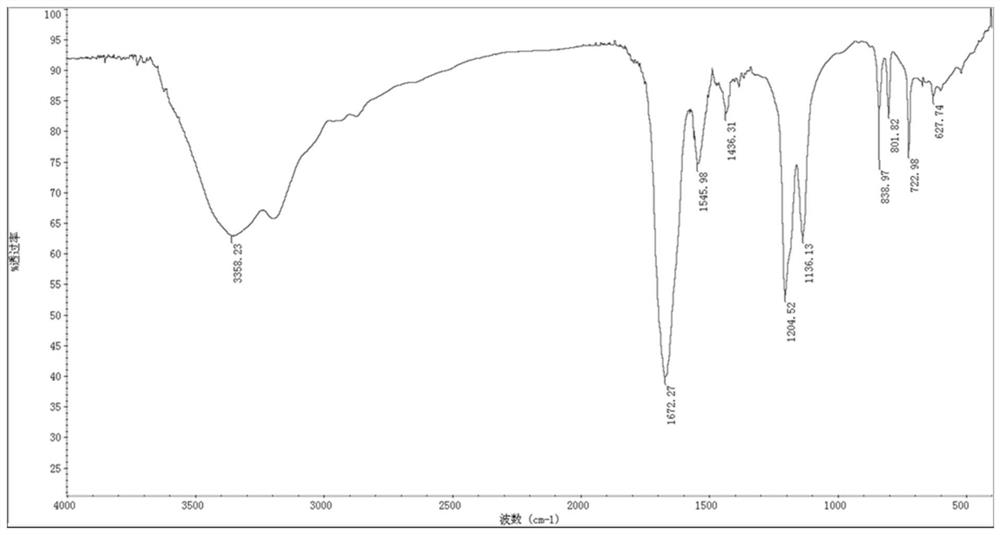
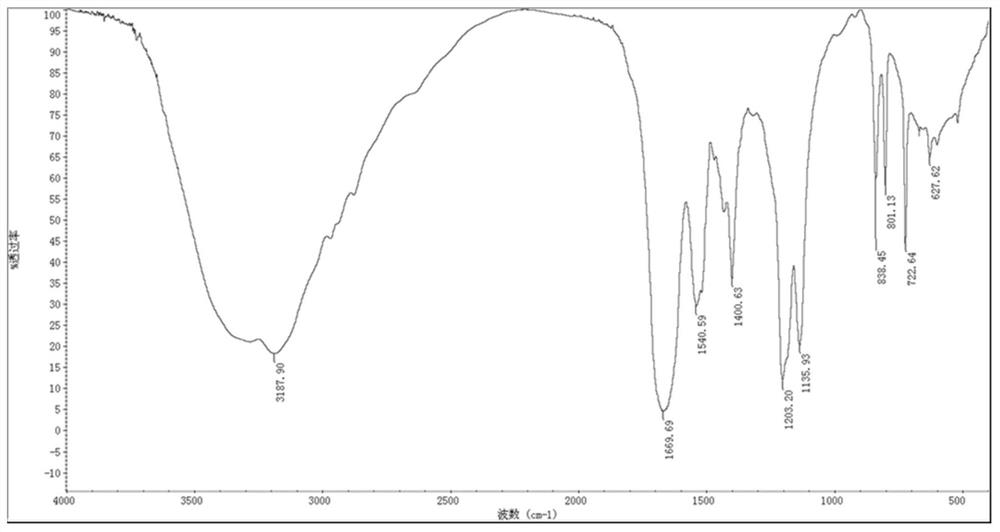
[0051] (1) Infrared spectrum identification
[0052] Take 1~2mg of solid dry samples of PHR and PHRD, grind them thoroughly in an agate mortar, add 400mg of dry KBr, and continue to grind until completely mixed, the particle diameter is about 2μm; take 100mg of the mixture and evenly spread it on a clean pressure film Inside, on the tablet press under the pressure of 29.4Mpa, press for 1min to make a transparent sheet; install the transparent sheet on the sample holder, place it at the sample cell of the spectrophotometer, and measure it from 4000cm -1 Sweep up to 400cm -1 .
[0053] It can be known from the structure of DR5 that there are free hydroxyl groups (3700cm -1 ~3500cm -1 ), free amino (3500cm -1 ~3300cm -1 ), associative amino group (3450cm -1 ~3200cm -1 ), carboxylic acid group (1900cm -1 ~1650cm -1 ), benzene ring (3000cm -1 , 1680cm -1 ~150...
Embodiment 3
[0057] Example 3 Construction of polylactic acid-polypeptide micelles co-loading DTIC and miRNA-34a
[0058] In this example, based on the targeted co-delivery of chemotherapeutic drugs and the gene drug carrier PHRD, the phacoemulsification method was used to prepare polypeptide micelles co-loaded with the chemotherapeutic drug dacarbazine (DTIC) and the gene drug miRNA-34a. The steps are as follows:
[0059] (1) Dissolve 3mg DTIC in 3mL methanol to prepare a 1mg / mL solution;
[0060] (2) Weigh 5 mg of PHRD, dissolve it in deionized water, and prepare a 1 mg / mL solution, add 3 mL of DTIC solution to the PHRD solution drop by drop, and ultrasonicate under ice bath conditions with a power of 100 W and a time of 30 s. 2 times;
[0061] (3) Quickly transfer the emulsified mixed solution to a magnetic stirrer for rapid stirring overnight, remove the methanol in the solution and collect the mixed solution, remove unencapsulated DTIC with a microporous filter membrane with a pore s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com