A kind of perakine reductase mutant and its application
A mutant, reductase technology, applied in the application of selective hydrogenation of α,β-unsaturated ketone double bonds, in the field of Perakine reductase mutants, which can solve the problem of unsatisfactory reaction yield and selectivity, and cannot be widely used , harsh reaction conditions and other problems, to achieve the effect of mild reaction conditions, environmental friendliness and high conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The mutation of embodiment 1Perakine reductase
[0024] The wild-type Perakine reductase expression plasmid has been constructed by the applicant's laboratory (Sun L, Ruppert M, Sheludko Y, Warzecha H, Zhao Y, J. Purification, cloning, functional expression and characterization of perakine reductase: the first example from the AKR enzyme family, extending the alkaloidal network of the plant Rauvolfia. Plant Mol Biol. 2008, 67(5): 455-67.) completed, its amino acid sequence As listed in SEQ ID NO.1, the nucleotide sequence is listed in SEQ NO.2. The above plasmid was heat-shocked and transformed into E.coli M15 competent cells for expression, and wild-type Perakine reductase was obtained after purification.
[0025] After consulting the literature and comparing the amino acid sequence of Perakine reductase with other members of the AKR superfamily, it was found that the amino acid at position 126 is the key site that determines the carbonyl or double bond of the AKR enz...
Embodiment 2
[0031] Expression and purification of embodiment 2.Perakine reductase mutant and glucose dehydrogenase
[0032] 1. Expression of Perakine reductase mutants
[0033] Inoculate the engineered bacteria expressing the Perakine reductase mutant successfully constructed above into LB medium containing 50 μg / ml ampicillin and 25 μg / ml kanamycin and culture with shaking at 37°C for 12 hours. Transfer to 1L LB medium containing the same concentration of ampicillin and kanamycin, when the optical density of the culture medium is OD 600 When it reaches 0.6, add isopropyl-β-D-thiogalactopyranoside (IPTG) with a final concentration of 0.3mM to induce 30h at 25°C, centrifuge the culture solution at 8000rpm for 10min, discard the supernatant medium, and take the bacteria The body was stored at -20°C until use.
[0034] 2. Expression of Glucose Dehydrogenase
[0035] In the preliminary research work, the applicant's laboratory has completed the construction of Bacillus megaterium glucose d...
Embodiment 3
[0038] Embodiment 3.Perakine reductase mutant activity detection
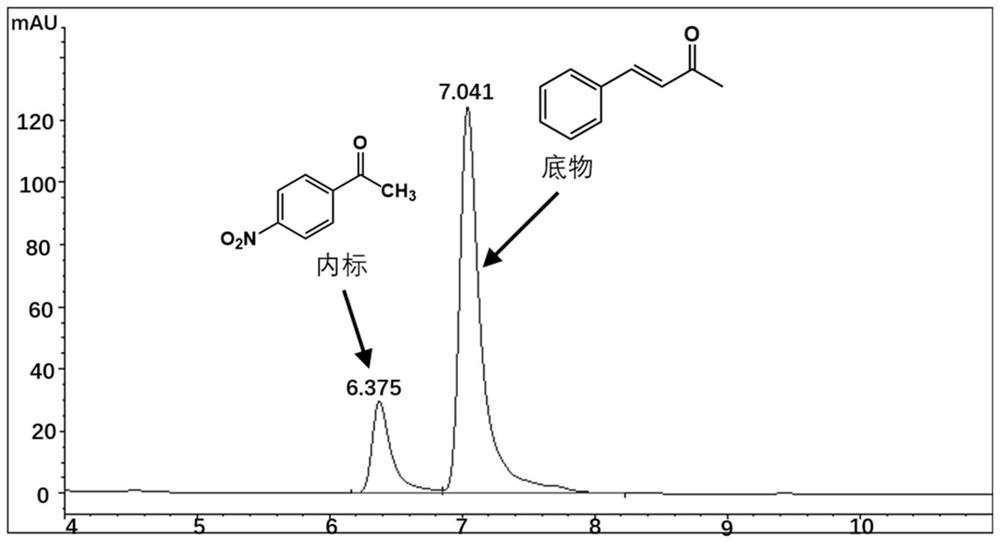
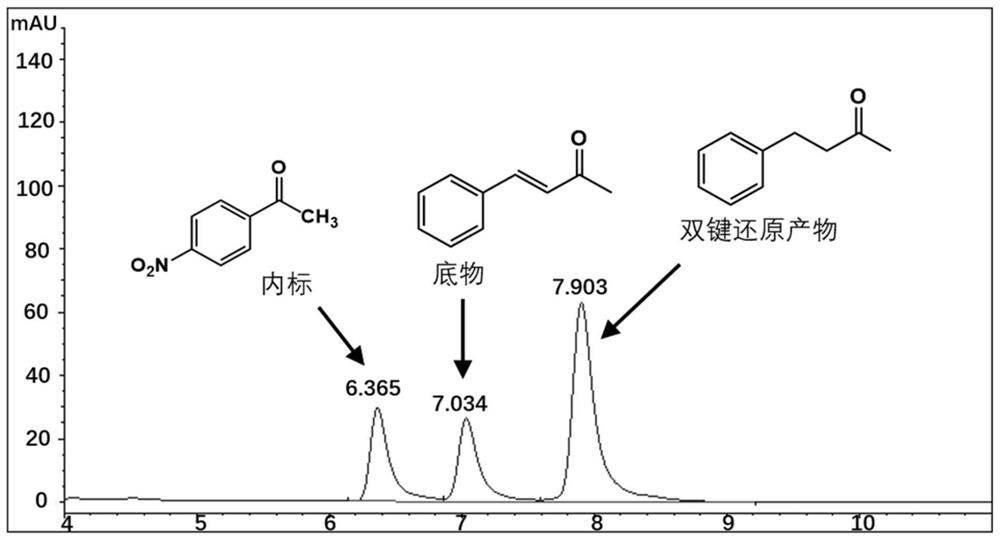
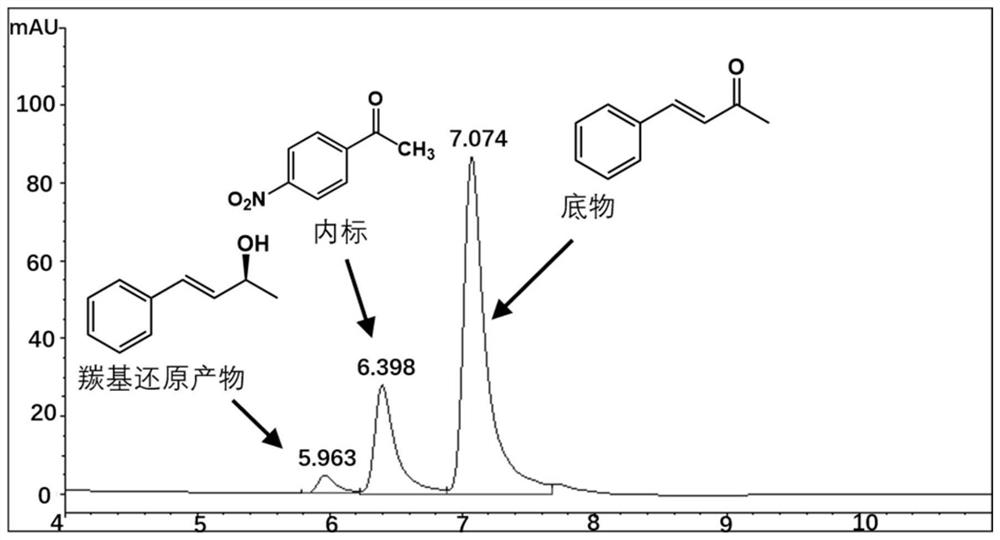
[0039] The Perakine reductase mutant and glucose dehydrogenase pure enzyme obtained in Example 3 were added to the reaction system at a concentration of 1 mg / ml, and 50 mM pH 7.0 Kpi was used as a buffer, and the final concentration was 0.8 mM respectively. Substrate benzylidene acetone, 1.2mM glucose, 0.02mM NADP + Shake at a constant temperature (175rpm) at 30°C for 10 hours, add an equal volume of methanol to terminate the reaction, centrifuge the reaction solution at 15,000rpm for 5 minutes, add internal standard p-nitroacetophenone, and inject high-performance liquid phase (HPLC) to detect the substrate and product Quantity is analyzed. The HPLC analysis method is: chromatograph Agilent high performance liquid chromatography 1100; chromatographic column Agilent 5HC-C18 250*4.6mm; column temperature 30°C; flow rate 1ml / min; detection wavelength 214nm; mobile phase: water 45%, acetonitrile 55%. The carbony...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com