Method for preparing high-purity crystallized D-Tagatose
A tagatose, cooling crystallization technology, applied in the preparation of sugar derivatives, chemical instruments and methods, monosaccharides, etc., can solve the problems of undeveloped research on the separation and purification of tagatose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
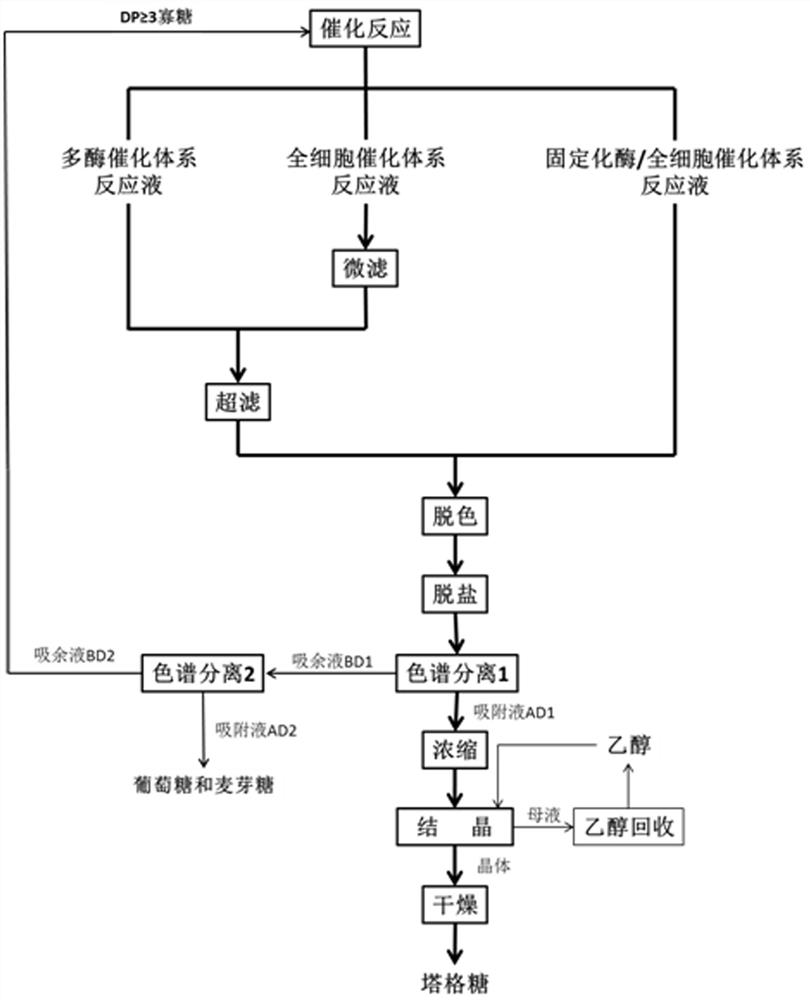
Image
Examples
Embodiment 1
[0034] Preparation of starting material for S1 purification:
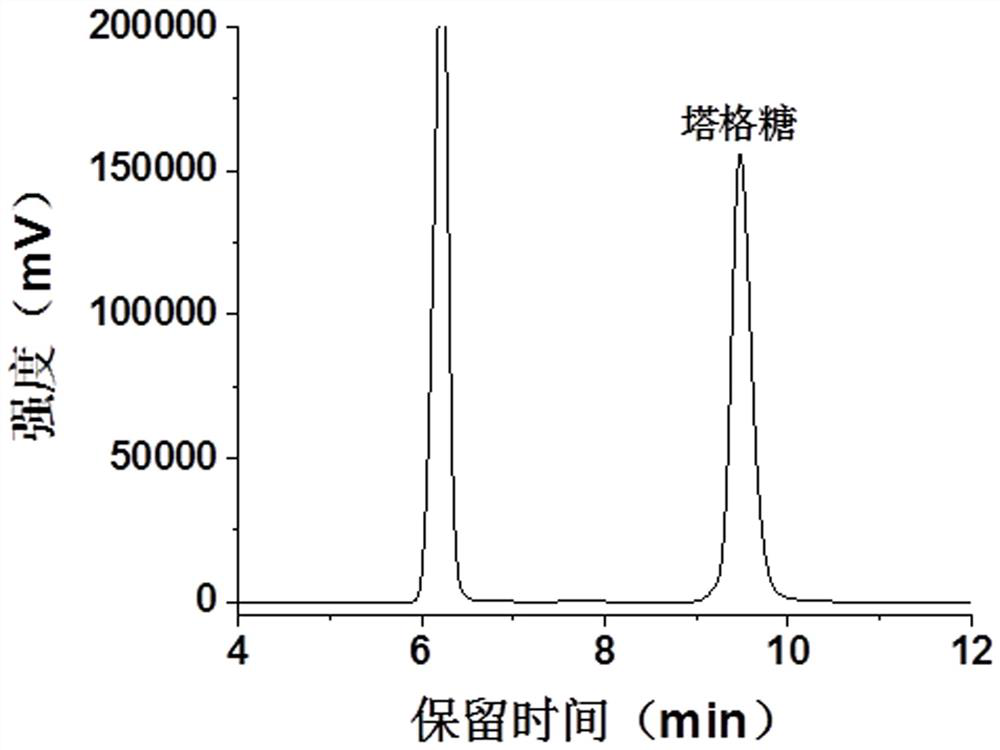
[0035] The multi-enzyme catalytic reaction system contains 30 mM phosphate buffer (pH 7.0), 5 mM divalent magnesium ions, the amount of α-glucan phosphorylase is 10 U / mL, the amount of glucose phosphomutase 10 U / mL, the dosage of glucose phosphate isomerase is 10 U / mL, the dosage of 6-phosphate tagatose epimerase is 15 U / mL, the dosage of 6-phosphate tagatose phosphatase is 15 U / mL, the dosage of starch debranching enzyme is 1 U / mL, soluble starch is 70 g / L, and the catalytic reaction is carried out at 70°C. After 36 hours of reaction, 10% sulfuric acid is added to terminate the reaction, and the detection is carried out by HPLC. Among them, the HPLC detection conditions are as follows: the chromatographic column is Bio-Rad HPX-87H; the flow rate is 0.6 mL / min; the column temperature is 60°C; the detector is a differential refractive index detector; the injection volume is 20 μL. The composition of the obtained re...
Embodiment 2
[0046] Preparation of starting material for S1 purification:
[0047] The whole-cell catalytic reaction system contains 30 mM phosphate buffer (pH 7.0), 5 mM divalent magnesium ions, the dosage of cells expressing α-glucan phosphorylase is 1.5 g DCW / mL, and the amount of cells expressing glucose phosphorylase is 1.5 g DCW / mL. 0.8 g DCW / mL for cells expressing bitase, 0.8 g DCW / mL for cells expressing glucose phosphoisomerase, and 6 g DCW for cells expressing tagatose-6-phosphate epimerase / mL, the amount of cells expressing 6-phosphate tagatose phosphatase is 6 gDCW / mL, the amount of cells expressing starch debranching enzyme is 0.8 g DCW / mL, maltodextrin 135 g / L, at 70 ℃ Catalyzed reaction, after 48 hours of reaction, the composition of the obtained reaction solution is as follows: tagatose 95.6 g / L, glucose 5.02 g / L, maltobiose 25.4 g / L, oligosaccharides (DP≥3) ~13 g / L , inorganic salts, pigments, cells and proteins. Carry out HPLC measurement by the same condition in embo...
Embodiment 3
[0059] Preparation of starting material for S1 purification:
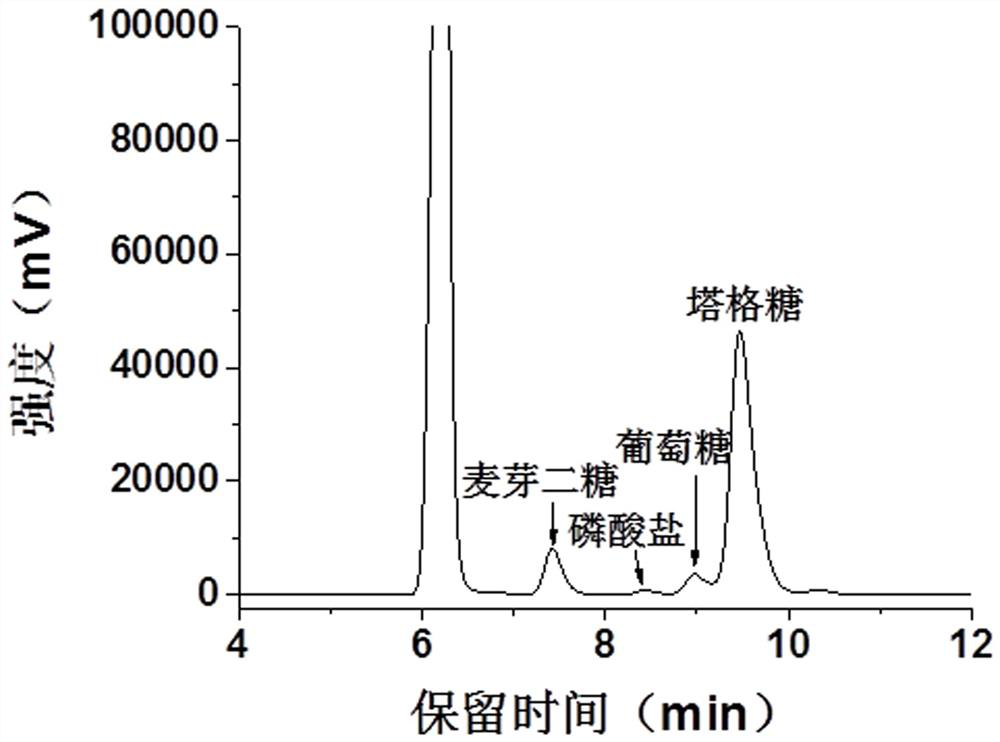
[0060] The immobilized whole-cell catalytic reaction system contains 30 mM phosphate buffer (pH 7.0), 5 mM divalent magnesium ions, and simultaneously immobilizes cells expressing α-glucan phosphorylase and expressing glucose phosphomutase Cells expressing glucose phosphate isomerase, cells expressing 6-phosphate tagatose epimerase, and cells expressing 6-phosphate tagatose phosphatase were used as catalysts, and the amount of immobilized cells was 20 g / L, maltodextrin 210 g / L, catalytic reaction at 70°C, after 48 hours of reaction, the composition of the obtained reaction liquid is as follows: tagatose 120 g / L, glucose 7.93 g / L, maltobiose 18.0 g / L, oligosaccharides (DP≥3) ~65 g / L, inorganic salts, pigments. The HPLC diagram of each component is shown in Figure 6 shown.
[0061] Since bacteria and proteins do not exist in the immobilized whole-cell catalytic reaction system, no bacteria and / or proteins are sep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com