Method for comprehensively recovering calcium and arsenic from calcium arsenate/calcium arsenite precipitate
A calcium arsenite and sediment technology, applied in the field of metallurgy, can solve the problems of high disposal cost, unstable chemical properties, unstable sediment, etc., and achieve the effect of small footprint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
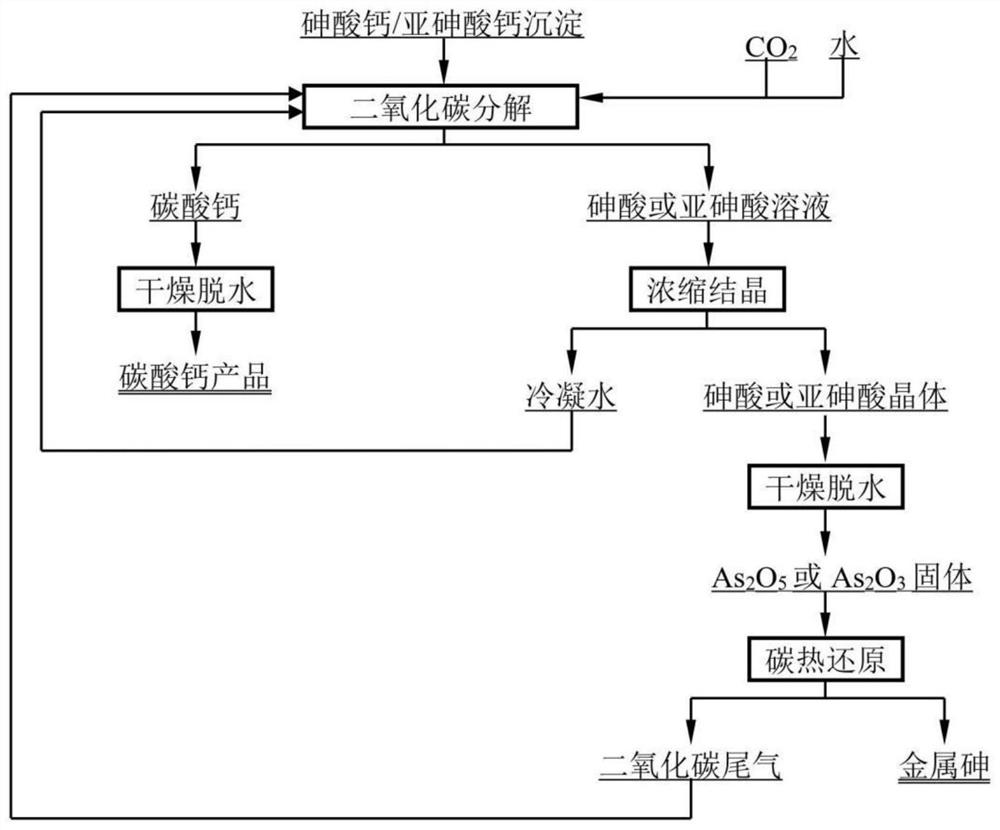
[0031]The raw material employed in this embodiment was added to the lead anode mud oxine soaking solution to the theoretical amount of 1.5 times lime, and the calcium arsenate, which was 27.75% arsenic.
[0032]100.00 g of calcium arsenate is added to 800 ml of water, mechanically stirred to form a slurry, and the molar ratio of calcium carbon dioxide gas, carbon dioxide and calcium carbon dioxide and calcium carbon dioxide and calcium carbon dioxide and calcium carbon dioxide is 5: 1, carbon dioxide. After the time was 480min, after the reaction, the mixed slurry was solid separated to give calcium carbonate and arsenic solution, and the arsenic content in the calcium carbonate was 0.4%; the arsenic solution was evaporated and crystallized, at the temperature of 1200 ° C, Time 90min, the reducing agent is coke, and the carbon heat reduction is carbonized under the conditions of C / As molar ratio, and 99.1% of metal arsenic is prepared.
Embodiment 2
[0034]The raw material employed in this example is to add arsenic wastewater to a theoretical amount of 1.3 times lime, and a mixture containing calcium arsenate is 25.13% of the mixture.
[0035]A mixture of 100.00 g of calcium containing mercycium arsenic acid was added to the water, mechanically stirred to form a slurry, with a carbon dioxide gas, carbon dioxide, calcium carbon dioxide and calcium carbonate and calcium carcium carbonate and calcium carbonate. When the ratio is 5: 1, the carbon dioxide is transferred for 240 minutes. After the reaction, the mixed slurry is liquidally solidified to obtain calcium carbonate and arsenic solution; the amount of arsenic in calcium carbonate is 0.2%; containing arsenic solution evaporation After crystallization, the temperature was 1100 ° C, the time was 60 min, and the reducing agent was graphite, and the reducing agent was carbonized under conditions of C / A molar ratio of 3 times, and 99.2% of metal arsenic was prepared.
Embodiment 3
[0037]The raw material employed in this example is to add the theoretical amount of 1.8-fold lime to the lead anode mud, which is obtained by a mixture of calcium arsenate, which contains 19.35%.
[0038]100.00 g of calcium arsenate is added to 800 ml of water, mechanically stirred to form a slurry, with a molar ratio of calcium carbon dioxide gas, carbon dioxide and calcium carbon dioxide and calcium carbon dioxide and calcium carbonate and calcium carbonate and calcium carbonate at 3:00 1. The carbon dioxide is 300 min. After the reaction, the mixed slurry is liquidally solidified to obtain calcium carbonate and arsenic solution; the amount of arsenic in calcium carbonate is 0.3%; the arsenic solution is evaporated by evaporation, The temperature is 900 ° C, the time is 150 min, the reducing agent is coal, and the reducing agent is carbon-heat reduction under conditions of C / A molar ratio of 2.0 times, and 99.0% metal arsenic is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com