A modified recombinant human type III collagen mature peptide containing hydroxyproline, its preparation method and application
A technology of collagen and hydroxyproline, applied in biochemical equipment and methods, chemical instruments and methods, animal/human protein, etc., can solve the problems of natural collagen gap, lack of post-modification of proline, etc., and achieve high molecular weight , good biological activity, and the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Construction of Pichia pastoris expression system containing proline hydroxylase gene
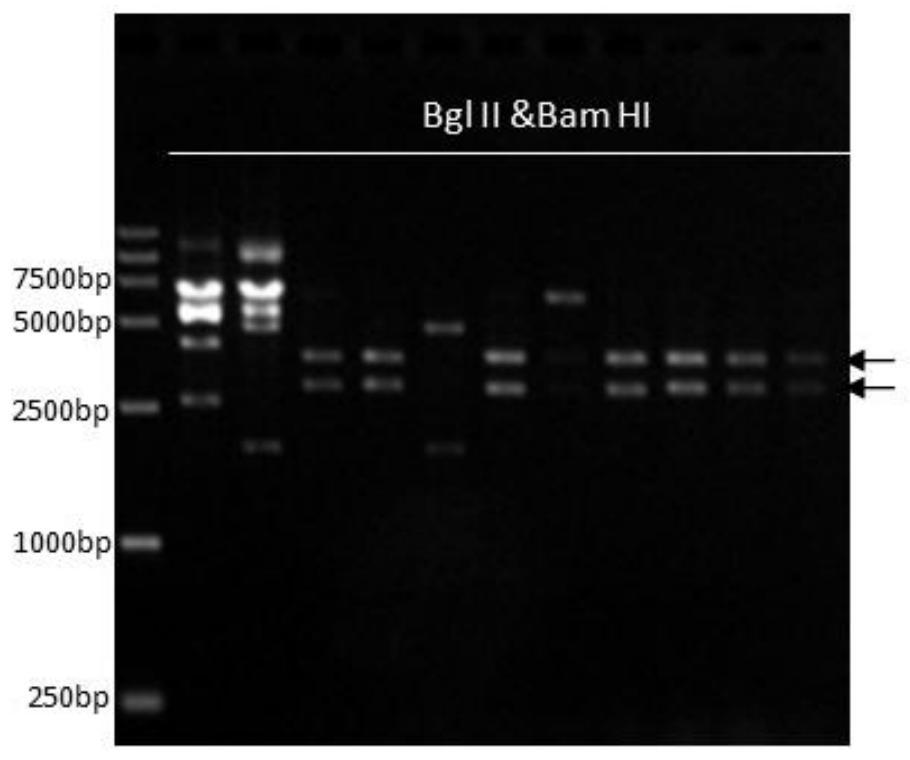
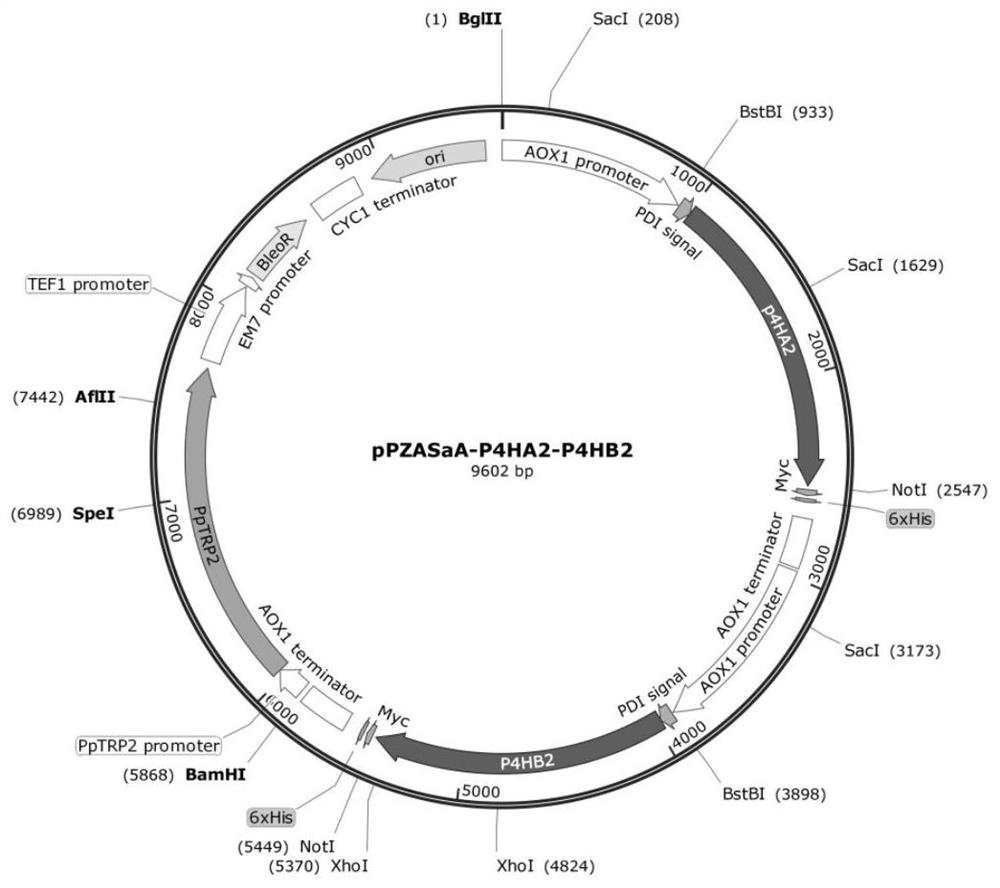
[0055] Using pPICZaA (purchased from Invitrogen) as the backbone, Pichia pastoris anthranilate synthase (Anthrailate synthase) gene was introduced to obtain the pPZSaA vector; then using the pPZSaA vector as the backbone, the P4HA2 and P4HB2 genes were respectively introduced into the multiple cloning sites, Obtain pPZSaA-P4HA2 and pPZSaA-P4HB2, and then connect the reading frames of the two through the BamHI / BglII restriction site to obtain the proline hydroxylase expression vector pPZAStA-P4HA2-P4HB2, and finally transform it into Pichia pastoris SMD1168 In this way, Pichia pastoris containing the proline hydroxylase gene was obtained. The detailed steps are as follows:
[0056] 1. Preparation of pPZSaA vector
[0057] (1) Utilize the Yeast Genome Purification Kit (purchased from TAKARA Company, product number 9082) to extract the Pichia pastoris genome, the steps are a...
Embodiment 2
[0089] Example 2 Expression of human type III collagen of different lengths in Pichia pastoris with proline hydroxylase gene
[0090] (1) The Pichia pastoris expression system containing the proline hydroxylase gene prepared in Example 1 is prepared into competent cells (the method is the same as in Example 1), and Pichia pastoris SMD1168 / pPZSaA-P4HA2-P4HB2 competent ;
[0091] (2) According to the protein resource database UniProt (URL https: / / www.uniprot.org / ) released the human type III collagen mature peptide sequence (P02461-1), combined with the codon preference of Pichia pastoris to optimize the design, and entrusted Shanghai Jierui Biotechnology Co., Ltd. to synthesize the Col3A1 gene, the corresponding amino acid sequence and nucleotide The sequences are as follows:
[0092] COL3A1 amino acid sequence:
[0093]QQEAVEGGCSHLGQSYADRDVWKPEPCQICVCDSGSVLCDDIICDDQELDCPNPEIPFGECCAVCPQPPTAPTRPPNGQGPQGPKGDPGPPGIPGRNGDPGIPGQPGSPGSPGPPGICESCPTGPQNYSPQYDSYDVKSGVAVGGLAGYPGPAGP...
Embodiment 3
[0113] Example 3 Production Process of Recombinant Human Type III Collagen
[0114] (1) Take the strains SMD1168 / pPZSaA-P4HA2-P4HB2, pPIC9k-COL3a1(428..1172) preserved at -80°C for activation, dip the bacterial liquid with an inoculation loop and streak on the YPD solid plate; culture at 30°C For 2 days, pick a single colony in YPD liquid medium, culture at 30°C, 200rpm for about 24h, until OD 600 Reaching the range of 5-40, this is the first-class seed liquid, and the seed liquid can be adjusted according to the size of the culture volume.
[0115] (2) Inoculate the above-mentioned prepared seed solution into a 50L fermenter according to the aseptic inoculation method (the initial fermentation BSM medium is 30L, the conditions are: the temperature is 28°C, the dissolved oxygen is not less than 20%, and the fermenter is added by feeding 25% ammonia water control pH is 5.5 ± 0.5.After the glycerin is exhausted, continue to add the sterilized glycerin that mass volume ratio is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com