Method for determining residual quantity of 10 aminoglycoside antibiotics in eggs
An aminoglycoside and determination method technology, applied in the field of trace veterinary drug residue detection, can solve problems such as contamination of a mass spectrometer detector, and achieve the effects of improving sensitivity, improving efficiency and good purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
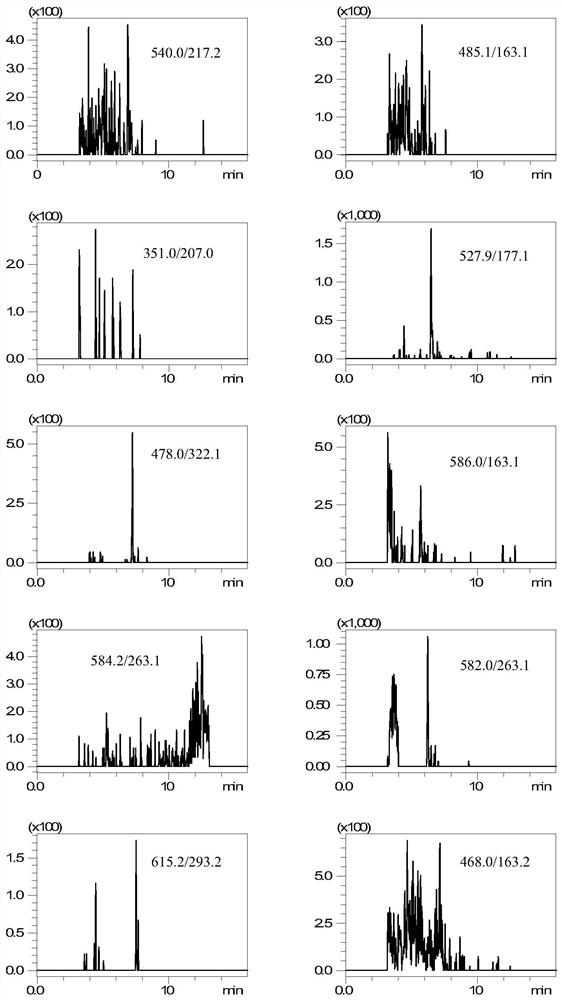
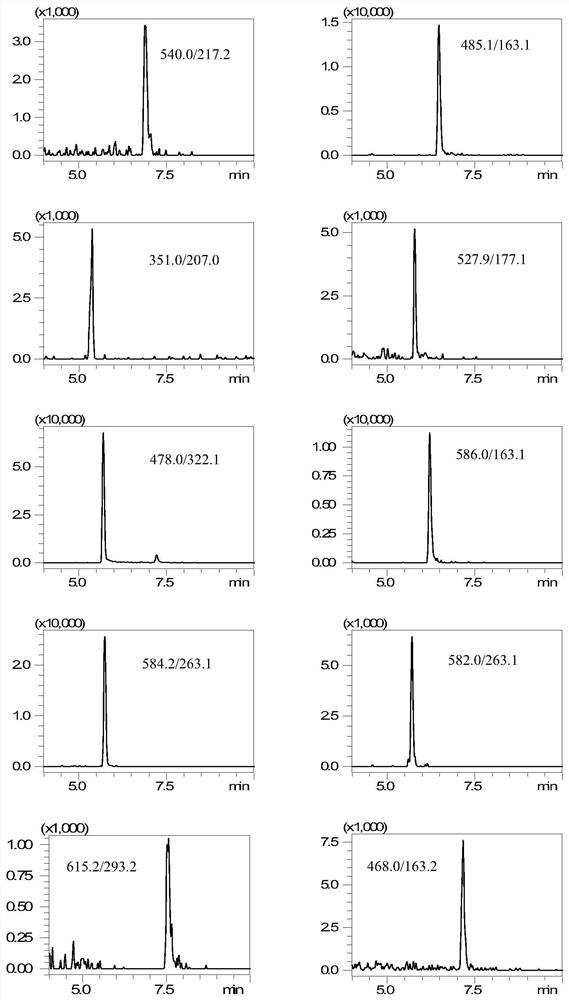
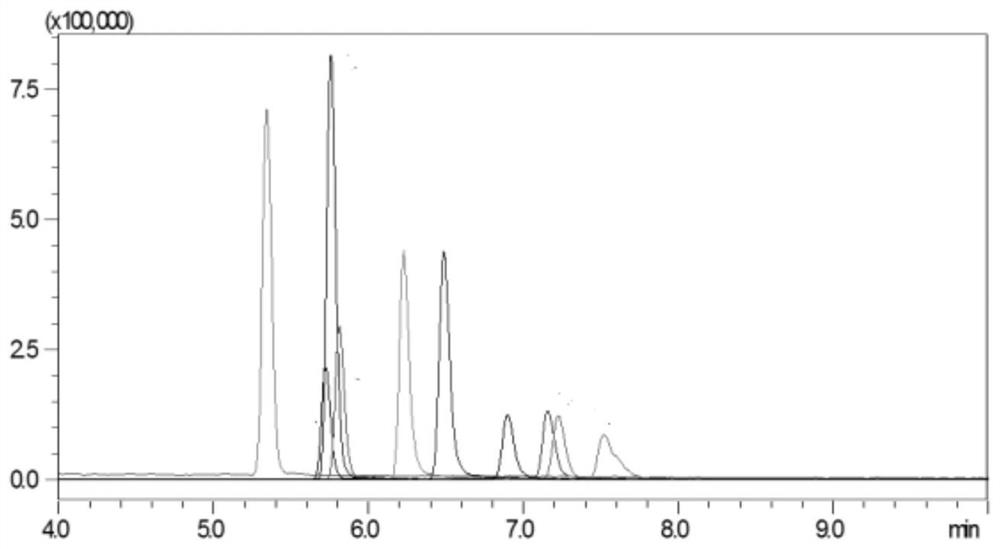
[0031] 1. Instruments and materials
[0032] Shimadzu LC / MS-8050 ultra-high performance liquid chromatography-tandem mass spectrometer (with electrospray ionization source); 3-18K desktop high-speed centrifuge; BT 125D electronic balance; MS3 vortex mixer; SB-800DTD ultrasonic cleaner; Milli-Q ultrapure water system.
[0033] Streptomycin, dihydrostreptomycin, hygromycin B, kanamycin, amikacin, tobramycin, apramycin, spectinomycin, neomycin, gentamicin standard Methanol; Acetonitrile; Formic acid; Ammonium formate; Trichloroacetic acid; Disodium EDTA; Hydrochloric acid; Sodium hydroxide; Ammonia; Ammonium acetate; Prime HLB solid-phase extraction cartridge (200 mg, 6 mL); Chromatography column (150×2.1mm, 5 μm).
[0034] 2. Pretreatment
[0035] 2.1 Standard stock solution and mixed standard intermediate solution
[0036] Accurately weigh and convert to 10 mg (accurate to 0.01 mg) streptomycin, dihydrostreptomycin, hygromycin B, kanamycin, amikacin, tobramycin, apramycin ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com