A kind of bio-based flame retardant magnolol epoxy monomer and its preparation method and application in flame retardant epoxy resin
An epoxy resin and bio-based technology, applied in the field of polymer chemistry, can solve the problems of relatively few studies on flame retardancy, and achieve the effect of simple and efficient curing process, wide sources, and high compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
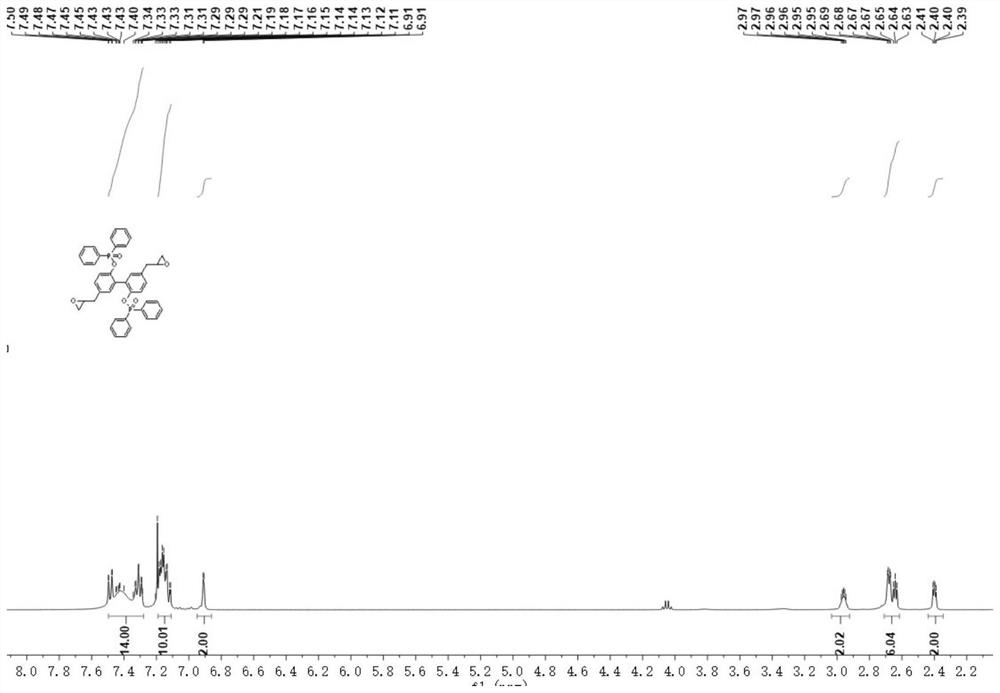
[0077] Example 1: Biologically flame retardant phenol epoxy monomer system:
[0078] First, in a clean three-neck bottle, a thick mougol (9.98 g, 37.5 mmol), sodium hydride (1.98 g, 82.5 mmol), add excess tetrahydrofuran (200 ml) under ice bath, so that it is fully dissolved and responding system The mixture was filled with nitrogen and held under ice bath for 15 min, slowly added diphenyl phosphon chloride (19.52 g, 82.5 mmol), stirring at room temperature for 3 h, and at room temperature, ethyl acetate and deionized water The solution ratio of 1: 1 (60 mL) was added to the reaction solution cooled under an ice bath, allowed for 30 min, add a small amount of saturated brine, ethyl acetate extraction reaction liquid, combined with organic phase, sodium anhydrous sodium sulfate Organic phase, filtration, discharilization of intermediate DBDBD; elution crude eluting elution (PE: EA, 2 / 1 to 1 / 2) using silica gel column chromatography (PE: EA, 2 / 1 to 1 / 2), and finally gave a white int...
Embodiment 2
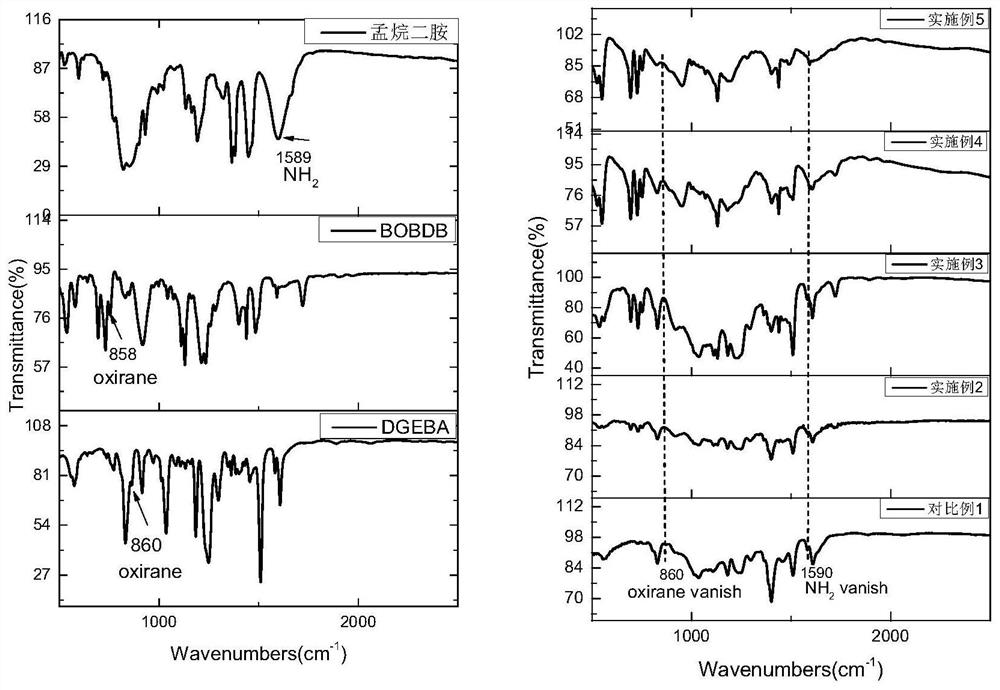
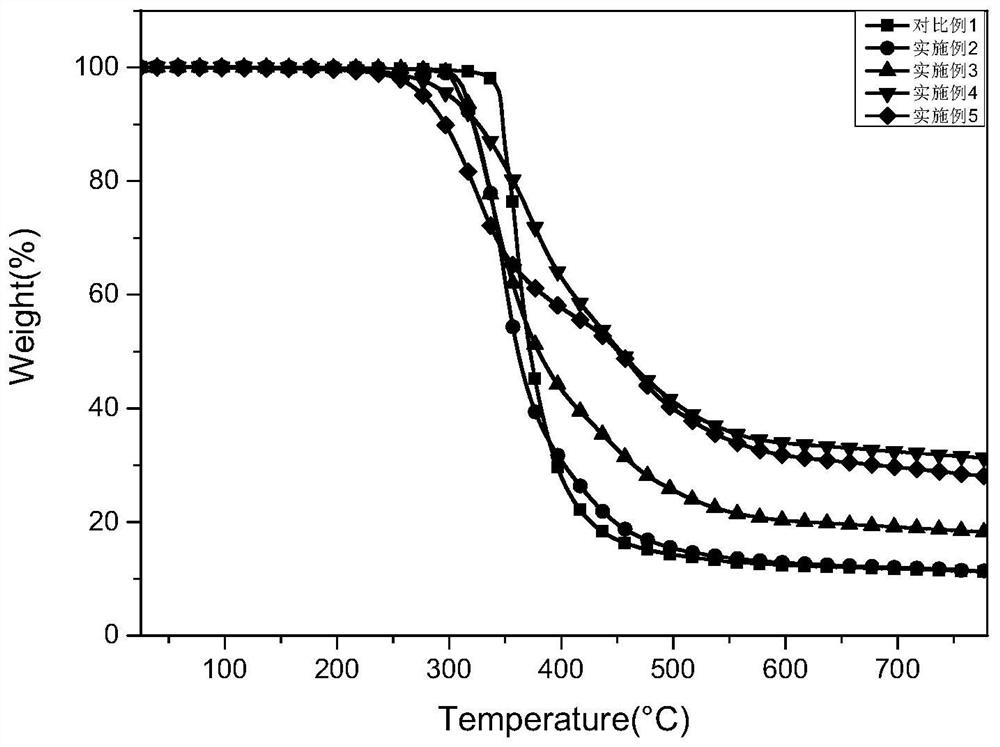
[0081] DGEBA monomers (0.1 g, 0.29 mmol), epoxy resin monomer BOBDB (0.037 g, 0.053 mmol), that is, 2% by weight of the total mass of the reaction system in the epoxy monomer BOBDB (0.037 g, 0.053 mmol), i.e. After nitrogen, after removal of oxygen components, the nitrogen atmosphere is added, and Menganentalamine (0.025 g, 0.145 mmol) is added, and the air is further removed, and the temperature is warmed to 55 ° C, which is melted (the molten process is transparently one) and mixed uniform , Cured from 200 ° C for 3 h to give a pale yellow transparent epoxy polymer. The minimum thermal release rate of the micro-combustion amount heat method (MCC) experiment was 431.95 W / g.
Embodiment 3
[0083] DGEBA monomers (0.1 g, 0.29 mmol), epoxy resin monomer BOBDB (0.11 g, 0.016 mmol), i.e., the epoxy monomer BOBDB (0.11 g, 0.016 mmol), that is, 4% by weight of the total mass of the C element in the epoxy resin monomer BOBDB, After nitrogen, after removal of the oxygen component, the nitrogen atmosphere is perhemened, and Menganentalamine (0.025 g, 0.145 mmol) is added, and the air is further removed, and the temperature is increased to 65 ° C to make both melt (molten transparent uniform) and mix well Curing from 200 ° C, cured 3 h, resulting in a pale yellow transparent epoxy resin polymer. The minimum thermal release rate of the experimental results of the micro-combustion amount (MCC) experiment was 303.24 W / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com