Drug delivery system of combining blood vessel blocking agent and double-drug-loading bionic liposome
A vascular blocking agent and double drug-loading technology, applied in the field of medicine, can solve the problems of unsatisfactory treatment effect of prodrugs, aggravating the degree of tumor hypoxia, etc., so as to reduce adverse reactions, reduce tumor microvessel density, and reduce the particle size of tumor cells. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
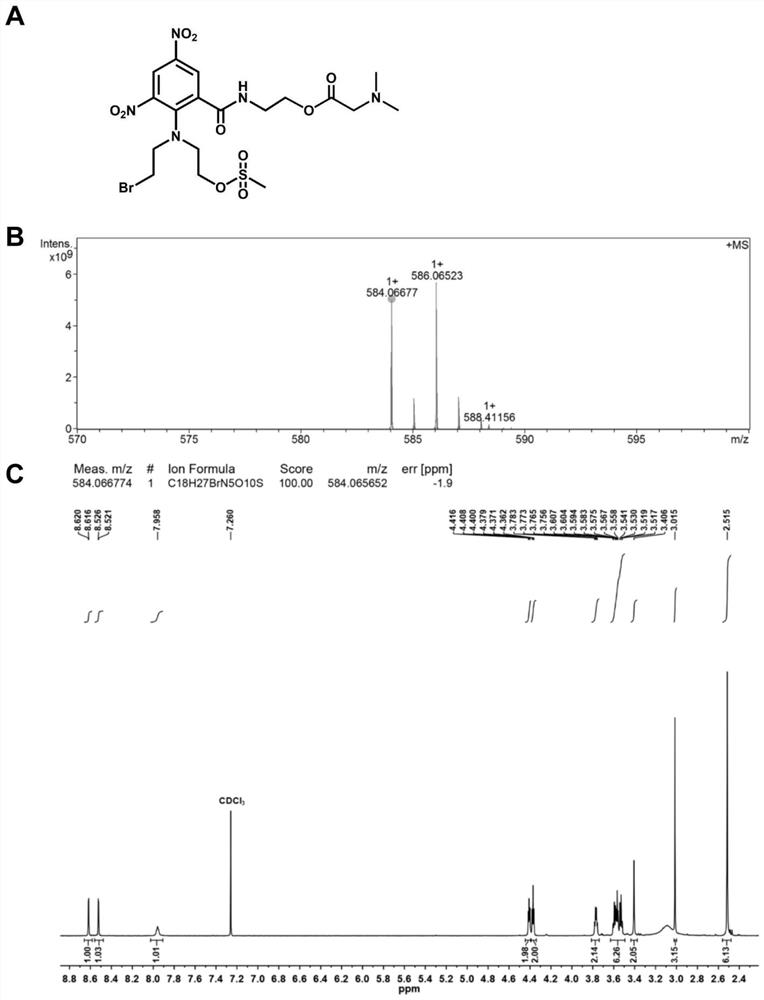
[0050] Example 1: Synthesis of the dinitrobenzamide mustard derivative DMG-PR104A (DP) whose base part is dimethylamino
[0051] Dissolve N,N-dimethylglycine (DMG, 69mg, 0.67mmol) in anhydrous acetonitrile and incubate in an ice bath at low temperature, and 2-(7-azabenzotriazole)-N,N,N', N'-Tetramethyluronium hexafluorophosphate (320mg, 0.84mmol) and N-methylmorpholine (180μL, 1.78mmol) were dissolved in a small amount of anhydrous acetonitrile and added dropwise to the above solution, and reacted under ice cooling for 1~ 2 hours. PR104A (500mg, 1mmol) was dissolved in a small amount of anhydrous acetonitrile and added dropwise to the reaction flask, reacted under nitrogen protection at room temperature for 24 hours, and separated and purified to obtain DP.
[0052] The structure of DP is as figure 1 As shown in (A), structure confirmed mass spectrum and 1 H NMR spectrum as figure 1 (B) As shown in (C), the solvent used for nuclear magnetic resonance is CDCl 3 , the analy...
Embodiment 2
[0054] Example 2: In vitro cytotoxicity test to screen the optimal ratio of photosensitizer PPa and hypoxia-activated prodrug DP for synergistic effect.
[0055] Cytotoxicity was assessed by MTT viability assay. 4T1 cells were seeded into 96-well plates at a density of 2500 cells per well and incubated overnight. After the cells adhered to the wall, the old culture medium was discarded, and serial dilutions containing PPa and DP were added to each well to treat the cells, and after incubation for 4 hours, irradiated with a 660nm laser (100mW / cm 2 , 2 minutes), and then continue to culture for 20 hours to examine the cytotoxicity. Using the Chou-Talalay method (also known as the median pharmacodynamic method), the combination index value (Combination Index, CI) of PPa and DP is used to quantitatively describe the synergistic effect of the combined application of the two drugs. , additive or antagonistic effects. The calculation formula of CI is as follows. When CI1, there is...
Embodiment 3
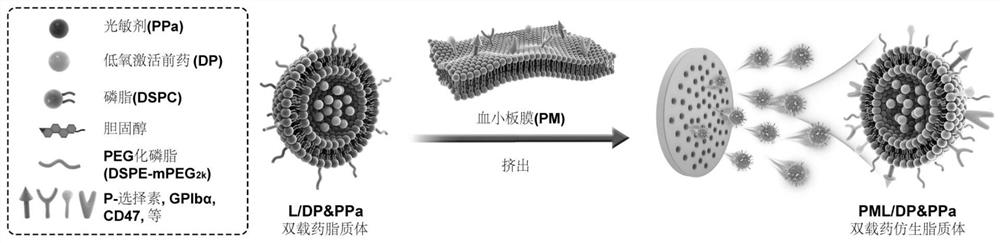
[0061] Example 3: Preparation of photosensitizer / hypoxia-activated prodrug double-loaded liposomes
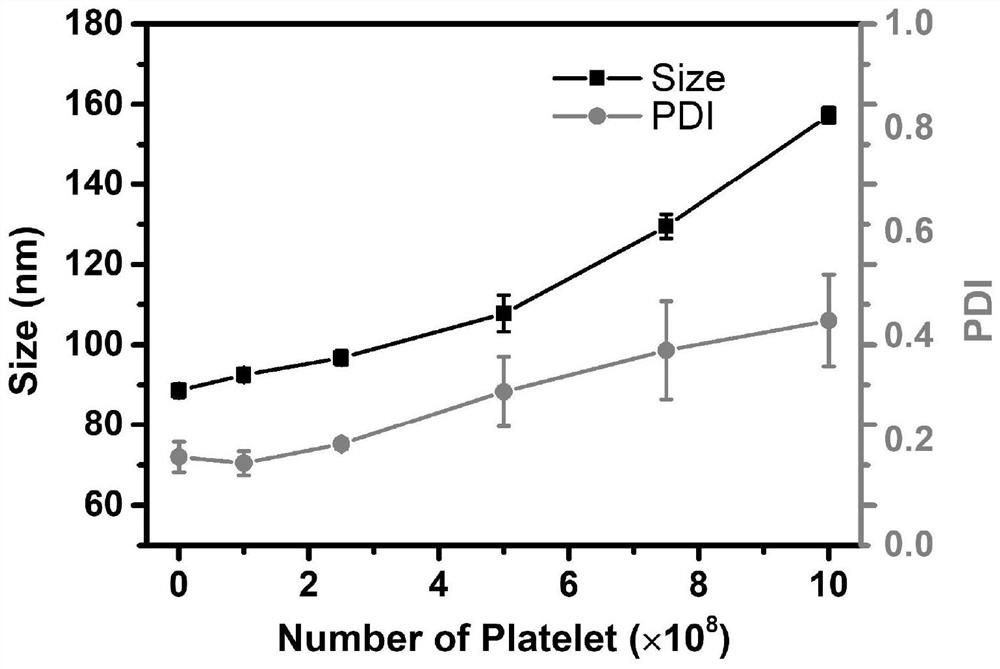
[0062] DSPC, cholesterol, DSPE-mPEG 2k Put it into an eggplant-shaped bottle according to the ratio of 3:1:0.05 (w / w), and add photosensitizer PPa, the ratio of PPa to total lipid is 1:200, dissolve it with a small amount of chloroform, and evaporate it under reduced pressure at 37°C Chloroform forms a lipid film. Add 300mM ammonium sulfate solution, hydrate at 65°C for 20 minutes, and sonicate for 10 minutes to prepare photosensitizer single-loaded liposomes. By means of agarose gel column chromatography, the external aqueous phase of the liposome was replaced with a mixed solution of 300mM sucrose and HEPES at pH 6, and the hypoxia-activated prodrug DP solution dissolved in ethanol was added dropwise, and the amount of DP and total lipid Drug-to-lipid ratio 1:10, incubate at 60°C for 20 minutes, stop drug loading in ice bath, and obtain photosensitizer / hypoxia-activated pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com