Alpha-L-rhamnosidase mutant enzyme, gene and expression preparation method
A technology of rhamnosidase and mutant enzymes, which is applied in the field of genetic engineering, can solve the problems of low yield and complex process, and achieve the effect of simple steps, broad application prospects, and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Construction of α-L-rhamnosidase mutant enzyme S303R encoding gene expression vector
[0021] Escherichia coli DH5α containing the WT (pPIC9K-r-Rhal) plasmid was inoculated and cultured in 30 mL LB liquid medium containing 1 mg / mL ampicillin resistance at 37° C. for 16 h. Use the small plasmid extraction kit to extract the WT ((pPIC9K-r-Rha1) plasmid according to the instructions. Use the site-directed mutagenesis kit of TOYOBO Biotechnology Co., Ltd. to construct mutants, and the steps are as follows:
[0022] ①Reverse PCR
[0023] Dilute the synthesized primers to 10 μM, adjust the concentration of the template plasmid pPIC9K-r-Rha1 to 50 ng / μL, and the reverse PCR reaction system is as follows: 17.5 μL of sterile water, 2.5 μL of 10× Buffer for iPCR; 2.5 μL μL of 2mMdNTPs; 0.75 μL of primers S303R-F, S303R-R; 0.5 μL of template plasmid; 0.5 μL of KOD-Plus. PCR reaction conditions: pre-denaturation at 94°C for 2 minutes, denaturation at 98°C for 10 sec...
Embodiment 2
[0032] Example 2: Expression and purification of α-L-rhamnosidase WT and mutant enzyme S303R using recombinant expression strains
[0033] Inoculate the strain prepared in Example 1 with 1% inoculum in 50 mL of YPD liquid medium for strain activation, shake culture at 30°C for 16 hours; then inoculate the activated strain into 100 mL of BMGY medium with 1% inoculum , 30°C, 200rpm, after culturing for 16 hours, measure and confirm that its OD600 reaches the range of 3.0-5.0; centrifuge for 10 minutes to collect all the bacteria, discard the supernatant, transfer all the bacteria to 100mL BMMY medium, 30°C, culture for 7 days, During the culture period, 0.5% anhydrous methanol was added to the medium every 24 hours; after the culture was finished, the supernatant was collected by centrifugation to be the enzyme solution.
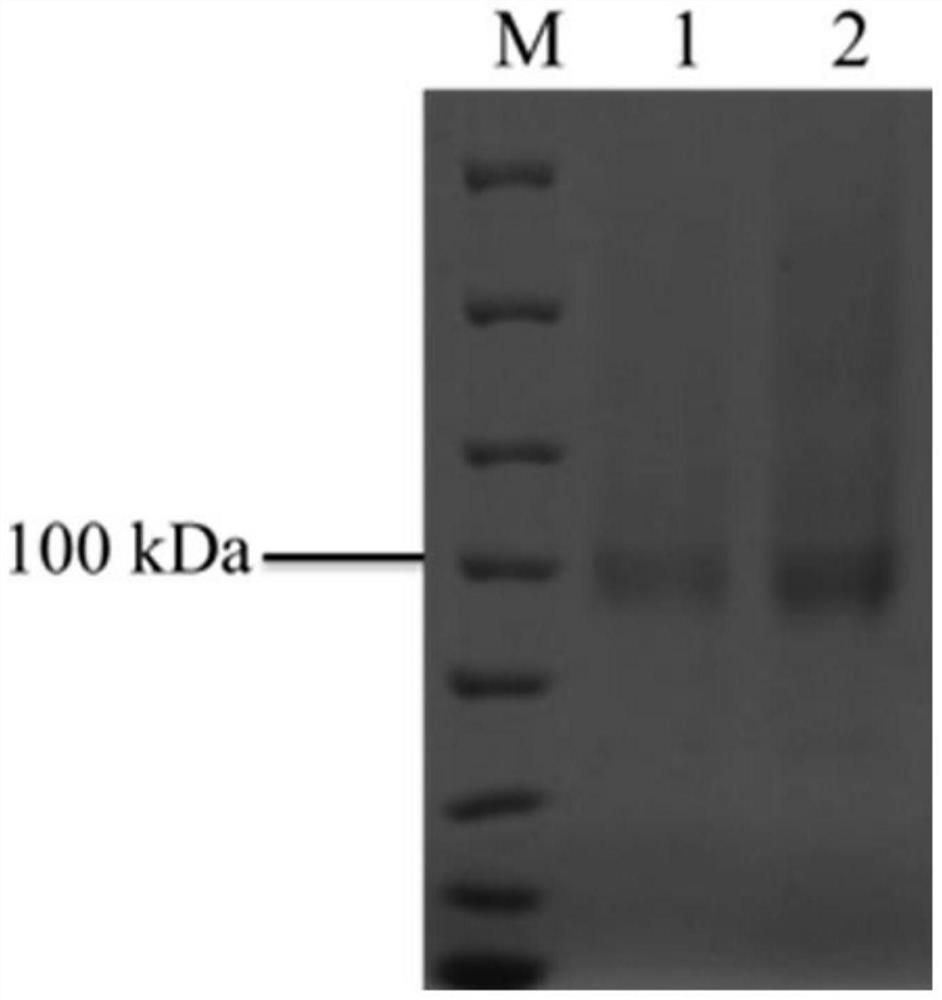
[0034] The crude enzyme solution of α-L-rhamnosidase WT and mutant enzyme S303R was collected, concentrated by ultrafiltration through a 30kDa membrane, and s...
Embodiment 3
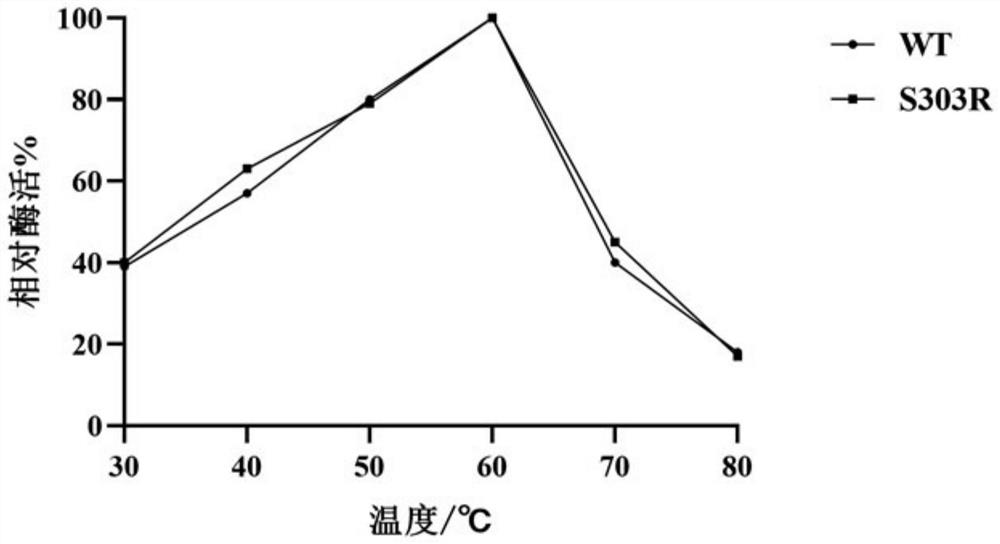
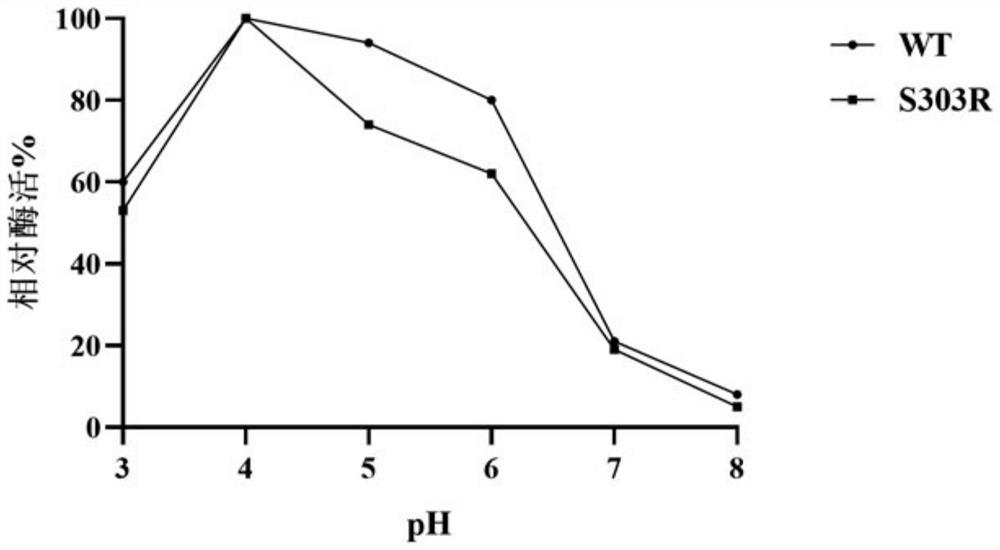
[0035]Example 3 α-L-rhamnosidase WT and S303R substrate specificity research
[0036] Using 0.5mmol / L naringin, hesperidin, rutin naringin, and citrusin as substrates, the substrate-specific hydrolysis rate of purified WT and S303R was determined, and the reaction system was: 1mL 0.5mmol / L Naringin, hesperidin, rutin naringin, citrusin, 980μL of 0.02mol / L citric acid-phosphate buffer (pH 4.0), incubate at 60°C for 10min, then quickly add 20μL of enzyme solution, After reacting for 10 minutes, put it into boiling water at 100° C. and boil for 10 minutes to terminate the reaction. Use a 1mL syringe to take 1mL of the reaction solution, inject it into a 1.5mL liquid phase bottle through a 0.22 μm water phase filter membrane, and finally measure the residual substrate concentration by Agilent 1260 liquid chromatography. The determination of the residual substrate concentration shows that the substrates of different enzymes The blank control of specific conversion rate was inactiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com