A kind of trifluoromethyl propenyl compound and its preparation method and application
A technology of trifluoromethylpropenyl and fluoromethylpropenyl, applied in the field of trifluoromethylpropenyl compounds and their preparation, can solve by-product halogen salt/high-salt wastewater, cumbersome post-reaction treatment, and reaction conditions Harsh problems, such as excellent stereoselectivity, tolerance to broad-spectrum functional groups, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) In a 10 mL Shrek tube, under an argon atmosphere, add 0.2 mmol of 2-hydroxy(phenyl) methyl methacrylate, 0.3 mmol of sodium trifluoromethanesulfinate, 0.008 mmol of 3,6,-di-tertiary Butyl-9-mesityl-10-phenylacridine-10-tetrafluoroborate, add 3mL of acetonitrile, stir the reaction under argon, room temperature and 18W blue light irradiation, the reaction equation is:
[0028]
[0029] (2) After the TLC monitoring reaction is complete, the solvent is removed with a vacuum rotary evaporator, the product is separated by thin layer chromatography, the developing solvent is petroleum ether / ethyl acetate system, and the product is a pale yellow liquid (E)-2-benzylidene -4,4,4-Methyl trifluorobutyrate, yield 71%.
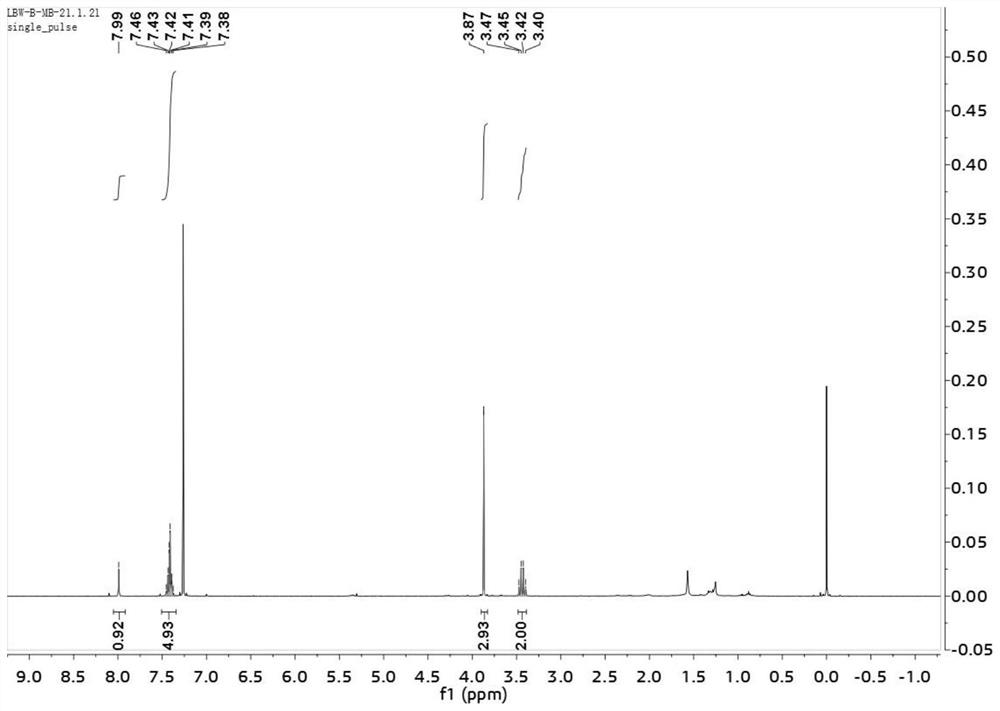
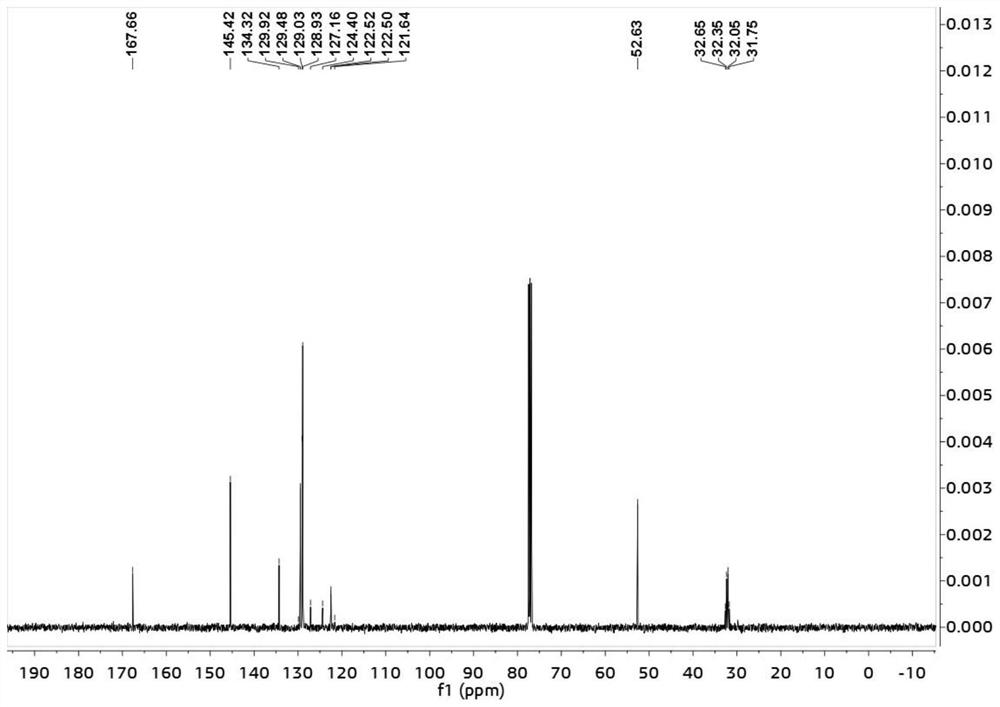
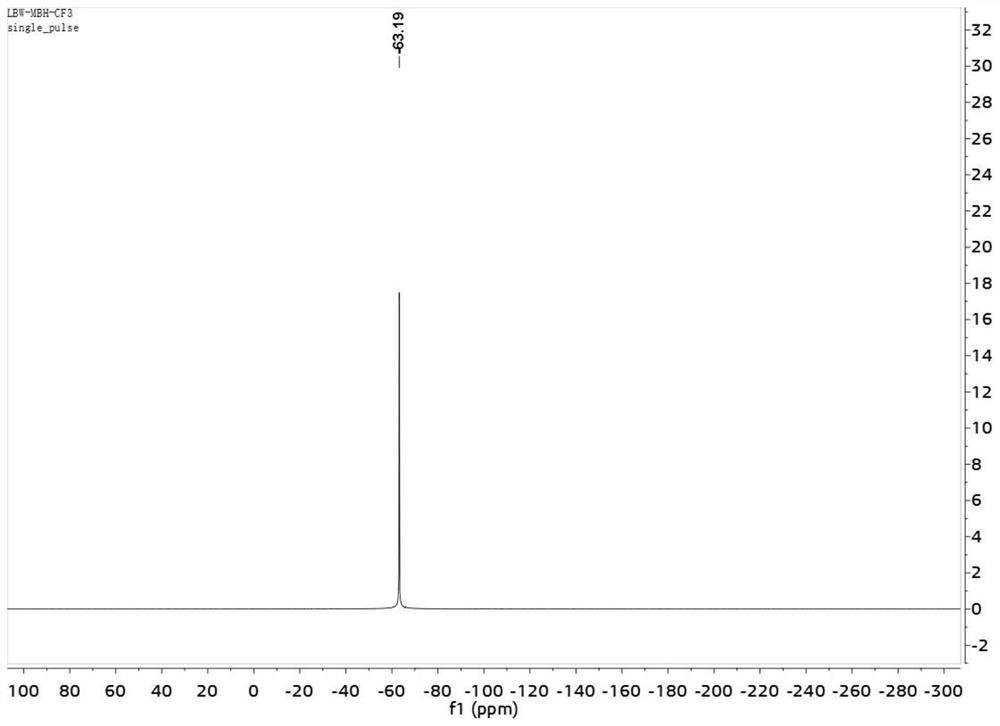
[0030] (E)-2-benzylidene-4,4,4-trifluorobutyric acid methyl ester was subjected to nuclear magnetic resonance test, such as Figures 1 to 3 shown, figure 1 is the H NMR spectrum of (E)-2-benzylidene-4,4,4-trifluorobutyric acid methyl ester; figure 2 is the...
Embodiment 2
[0032] (1) In a 10 mL Shrek tube, under an argon atmosphere, add 0.4 mmol of 2-hydroxy(2,4-dichlorophenyl)methylcyclohex-2-en-1-one, 0.6 mmol of trifluoromethyl Sodium sulfinate, 0.016mmol 3,6,-di-tert-butyl-9-mesityl-10-phenylacridine-10-tetrafluoroborate, add 6mL acetonitrile, under argon, room temperature and 18W blue light The reaction is stirred under irradiation, and the reaction equation is:
[0033]
[0034] (2) after the TLC monitoring reaction is complete, the solvent is removed with a vacuum rotary evaporator, the product is separated by thin layer chromatography, and the developing agent is a petroleum ether / ethyl acetate system, and the product is a pale yellow liquid (E)-2-(2, 4-Dichlorobenzylidene)-3-(trifluoromethyl)cyclohexan-1 one, 74% yield.
Embodiment 3
[0036] This embodiment is basically the same as the above-mentioned embodiment 1, except that 10 mmol of 2-hydroxy(phenyl) methyl methacrylate, 15 mmol of sodium trifluoromethanesulfinate, 0.4 mmol of 3 ,6,-di-tert-butyl-9-mesityl-10-phenylacridine-10-tetrafluoroborate, add 150 mL of acetonitrile, stir the reaction under argon, room temperature and 18W blue light irradiation to obtain ( E) -2-benzylidene-4,4,4-trifluorobutyric acid methyl ester 1.76 g, yield 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com