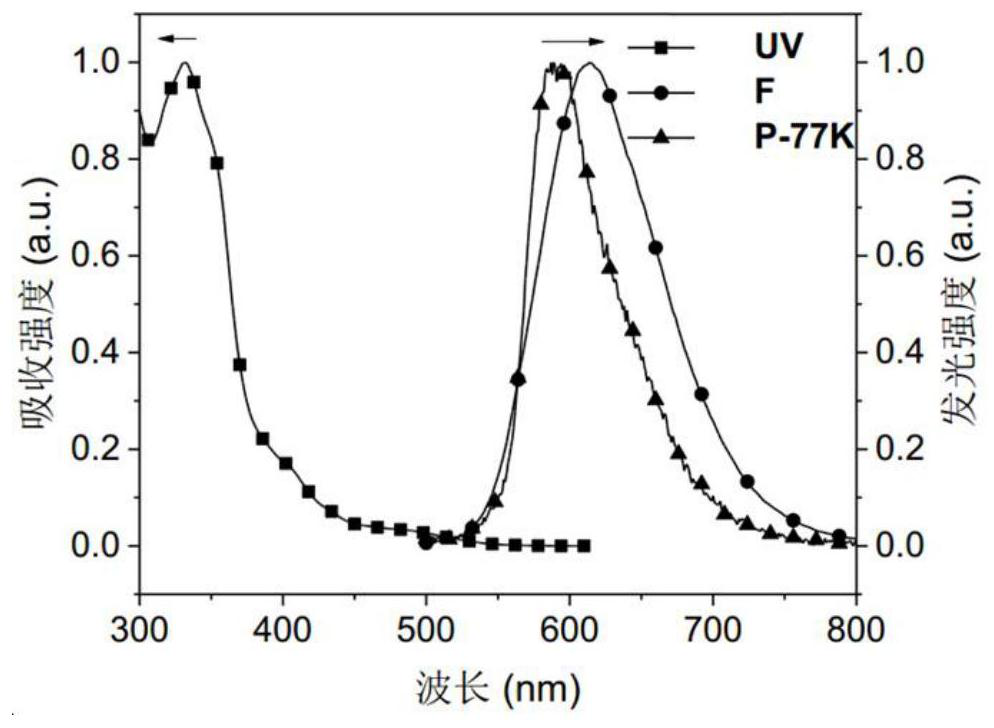
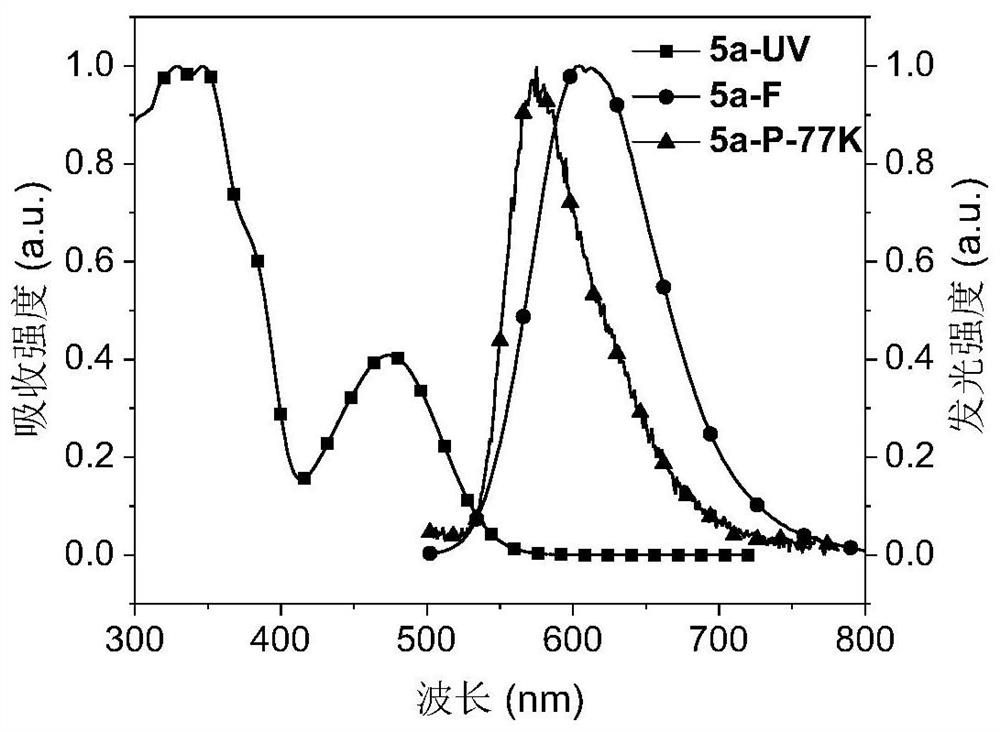
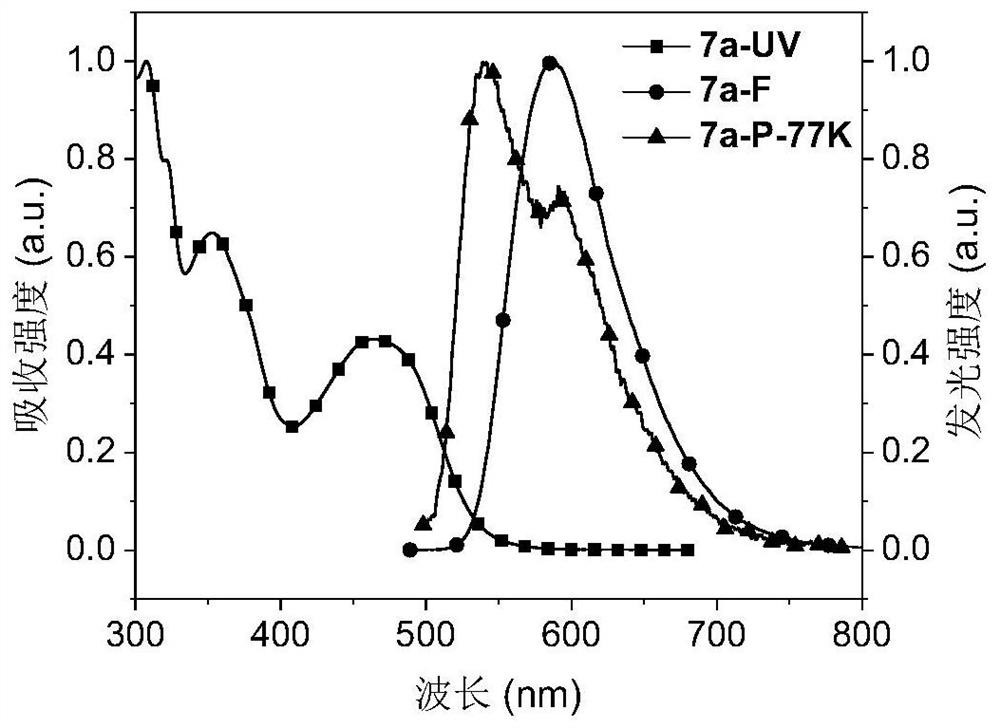
Red-light thermally-induced delayed fluorescence material and preparation method thereof, and organic electroluminescent device
A technology of delayed fluorescence and fluorescent materials, which is applied in the field of luminescent materials, can solve the problems of unfavorable development of large-size OLED full-color display, strong intermolecular force, and low luminous efficiency, so as to suppress non-radiative transitions, improve luminous performance, and high The effect of luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The present invention also provides a preparation method of the red light heat-induced delayed fluorescent material described in the above technical solution, comprising the following steps:
[0067] a) bromoindanone X undergoes an Aldol condensation reaction to form a tribromotriindene intermediate Y;
[0068] b) The tribromotrisindene intermediate Y reacts with aryl borate Z to form a multi-armed star precursor L;
[0069] c) The multi-armed star precursor L undergoes an oxidation reaction to form a red photothermally induced delayed fluorescent material represented by formula (I);
[0070]
[0071] In the formula Z, the aryl Ar- is the unit.
[0072] Regarding step a): bromoindanone X undergoes an Aldol condensation reaction to form a tribromoindan intermediate Y.
[0073] In the present invention, the bromoindanone is shown in formula X:
[0074]
[0075] Preferably, the bromoindanone X is 5-bromoindanone or 6-bromoindanone. When 6-bromoindanone is used ...
preparation example 1
[0116] Intermediate Preparation Example 1: Preparation of 3,8,13-tribromotripolyindene intermediate (i.e. 3,8,13-substituted type, i.e. Y-M) synthetic route is as follows:
[0117]
[0118] The preparation process is as follows:
[0119] Dissolve 6-bromoindanone (3.0g, 14.2mmol), p-toluenesulfonic acid monohydrate (9.5g, 49.9mmol) in dry o-dichlorobenzene (45mL), add propionic acid (3.7mL) under stirring at room temperature ), the system was heated to 135°C for reflux reaction for 72h. After the reaction, cool to room temperature, pour the reaction solution into 100mL methanol, and adjust the pH of the system to neutral with 1M NaOH aqueous solution. At this time, a large amount of precipitates will precipitate out, filter, and wash the filter cake with methanol and ethanol in sequence to obtain the intermediate Body Y-M, light yellow solid 2.3g, yield 84%.
preparation example 2
[0120] Intermediate Preparation Example 2: Preparation of 2,7,12-tribromotripolyindene intermediate (i.e. 2,7,12-substituted type, denoted as Y-N)
[0121] The synthetic route is as follows:
[0122]
[0123] The preparation process is as follows:
[0124] According to the preparation process of Intermediate Preparation Example 1, the difference is that the raw material 6-bromoindanone is replaced by 5-bromoindanone. As a result, the product intermediate Y-N was obtained as a light yellow solid with a yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com