Oral care product
A technology for oral care and composition, which is applied in the field of oral care compositions, and can solve problems such as irritation, reduction of formulation efficacy, and poor affinity for tooth surfaces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0152] 1.1.1. Composition 1.1, wherein the acid to form a salt of the alkaline amino acid is a carboxylic acid.
[0153] 1.1.2. Composition 1.1, where the acid to form a salt of the alkaline amino acid is an inorganic acid, wherein the inorganic acid is not phosphoric acid or sulfuric acid.
[0154] 1.1.3. Composition 1.1.1, wherein the carboxylic acid is selected from the group consisting of citric acid, lactic acid, glycolic acid and acetic acid, succinic acid, fumaic acid or a combination thereof.
[0155] 1.1.4. Composition 1.1.2, wherein the inorganic acid is not phosphoric acid or sulfuric acid, for example, when the particles are calcium carbonate, the inorganic acid is sodium hydrochloric acid or sodium pyrophosphate.
[0156] 1.1.5. Composition 1.1.2, wherein the inorganic acid is not phosphoric acid or sulfuric acid, wherein the inorganic acid is hydrochloric acid or sodium pyrophosphate only when calcium carbonate is present.
[0157] 1.1.6. Composition 1.1.2, there is a...
Embodiment 1
[0422] Example 1: Preparation
[0423] Optimized arginine toothpaste preparations were prepared using the following ingredients:
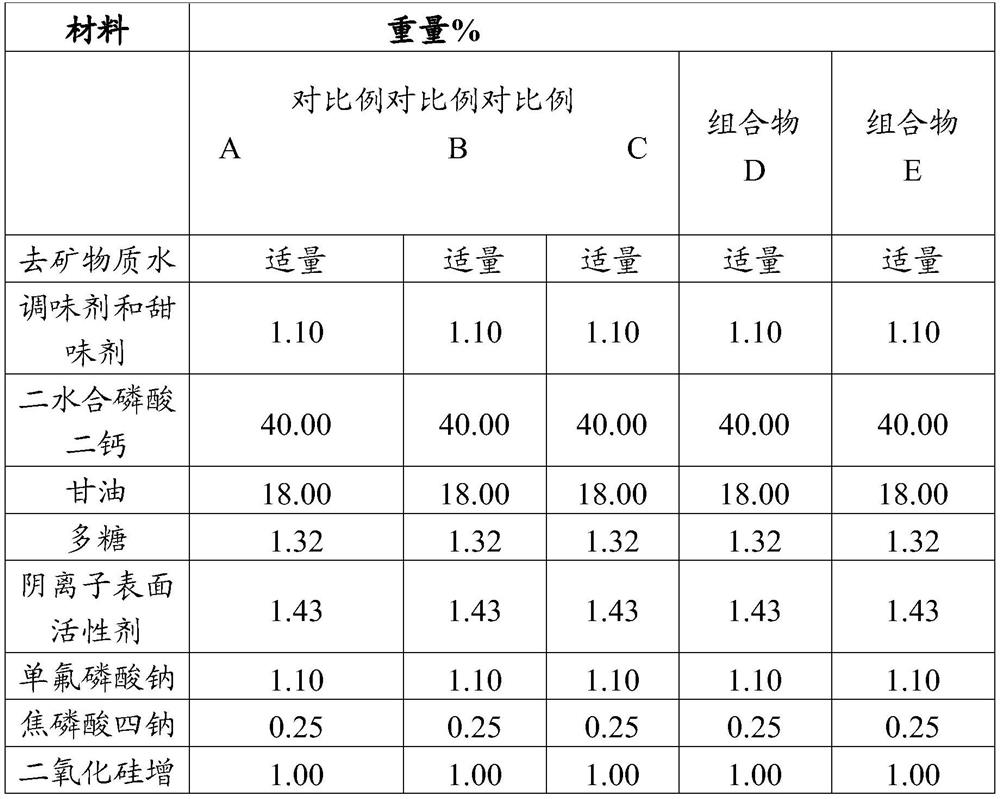
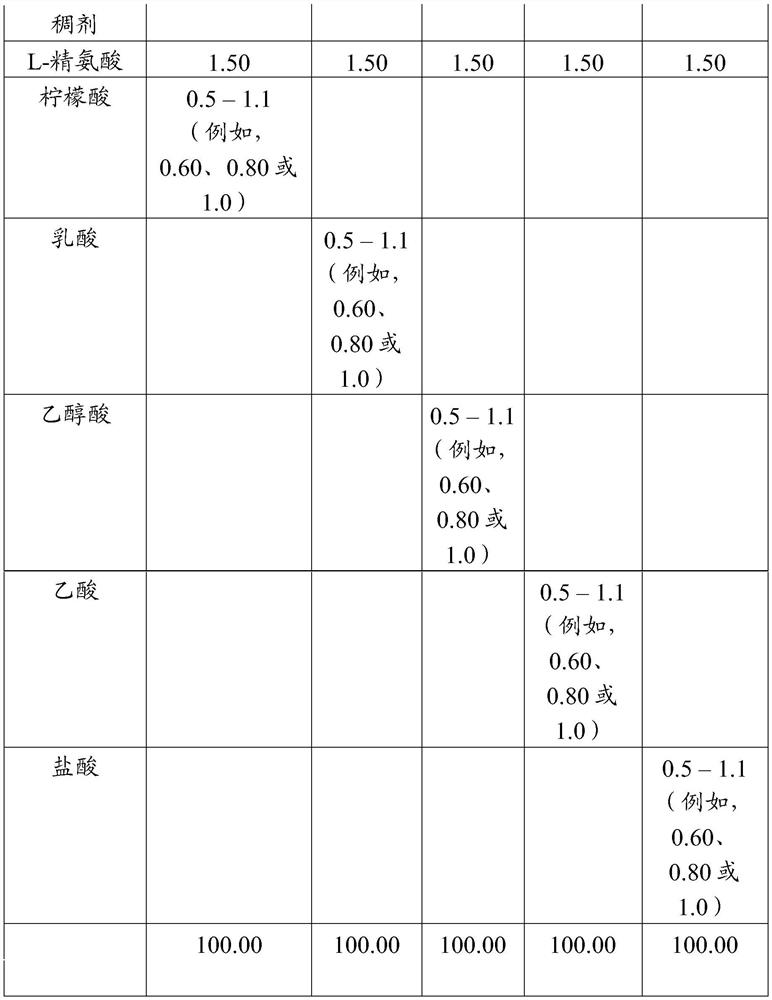
[0424] Table A
[0425]
[0426]
[0427] Table A (continued)
[0428]
[0429]
[0430] Table B
[0431]
[0432]
[0433]
[0434] Table B (continued)
[0435]
[0436]
Embodiment 2
[0438] Acids produced by oral bacteria can cause dental enamel dehydration and the development of dental caries over time. The arginine from the beneficial oral symbiotic via the decomposition metabolism of the arginine dethozyme pathway causes the alkali to produce (ammonia), which contributes to the prevention of neutralizing and tooth decaying in the plaqueous acid. Evaluation of Gordococcus Soclas in vitro - a known arginine decomposing bacteria to metabolize different formulations in different formulations. The ammonia produced by the split permeability method of 670 nm using a colored ammonia test method.
[0439] As shown in Table C below, in the step-by-ammonia production of ammonia production, an arginine-containing dentifrust formulation results in a negative control-containing 0.6% phosphoric acid but unvinoic acid solution significantly higher solution. Ammonia production. Surprisingly, the choice of acid used in the formulation seems to play in the ability of Gordonyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| oil absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com