Composition suitable for recovery phase of hyperlipidemia type acute pancreatitis patient and preparation method thereof
A technology for acute pancreatitis and hyperlipidemia, which is applied in the field of composition and its preparation for patients with hyperlipidemic acute pancreatitis in the recovery period, and can solve the problem of reducing blood lipid levels in the body, increasing blood lipid levels in patients, and exacerbating the course of pancreatitis To achieve the effect of promoting the recovery of pancreatic function, promoting the recovery of patients, and being easy to dissolve in fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also discloses a method for preparing a composition suitable for patients with hyperlipidemic acute pancreatitis in the recovery period, which includes the following steps:
[0044] Step 1. Raw material pretreatment: dry polypeptide powder, carbohydrates, fat powder and micronutrient raw materials at 60°C±2°C for 1.5-2h, detect moisture, dry and wet weight 1%-3%, pulverize, pass through 60-100 mesh sieve ,spare;
[0045] Step 2. Weighing and mixing: Weigh each component according to parts by mass; mix the weighed polypeptide powder, carbohydrate, and fat powder evenly to obtain mixture A; mix the weighed micronutrients in a gradient-increasing manner Uniformly, mixture B is obtained;
[0046] Step 3. Mixing: Mix mixture A and mixture B for 20-150 minutes, add phospholipids and glidants until mixture C is obtained, and pass through a 60-100 mesh sieve;
[0047] Step 4, Sterilization: Sterilize the sieved mixture C, so as to ensure that it can be u...
Embodiment 1
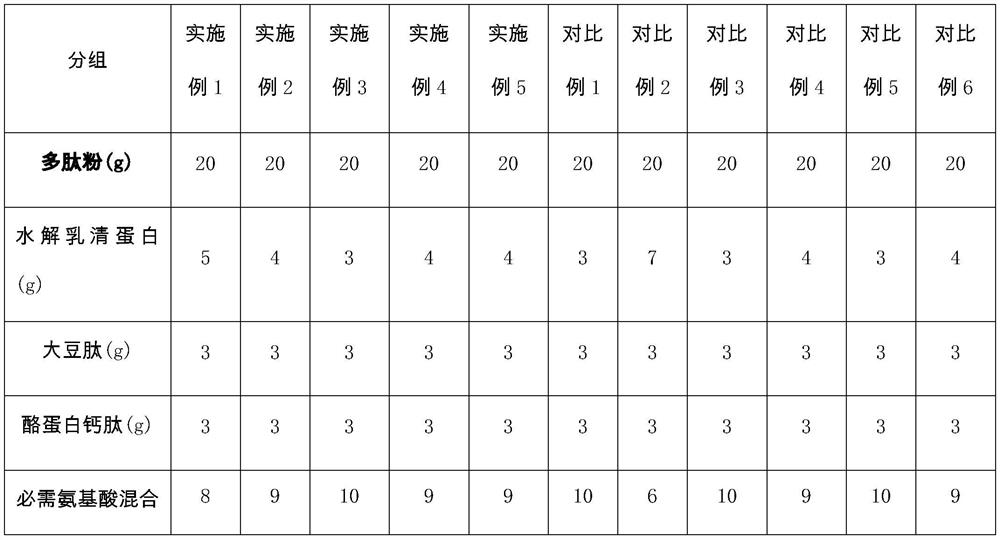
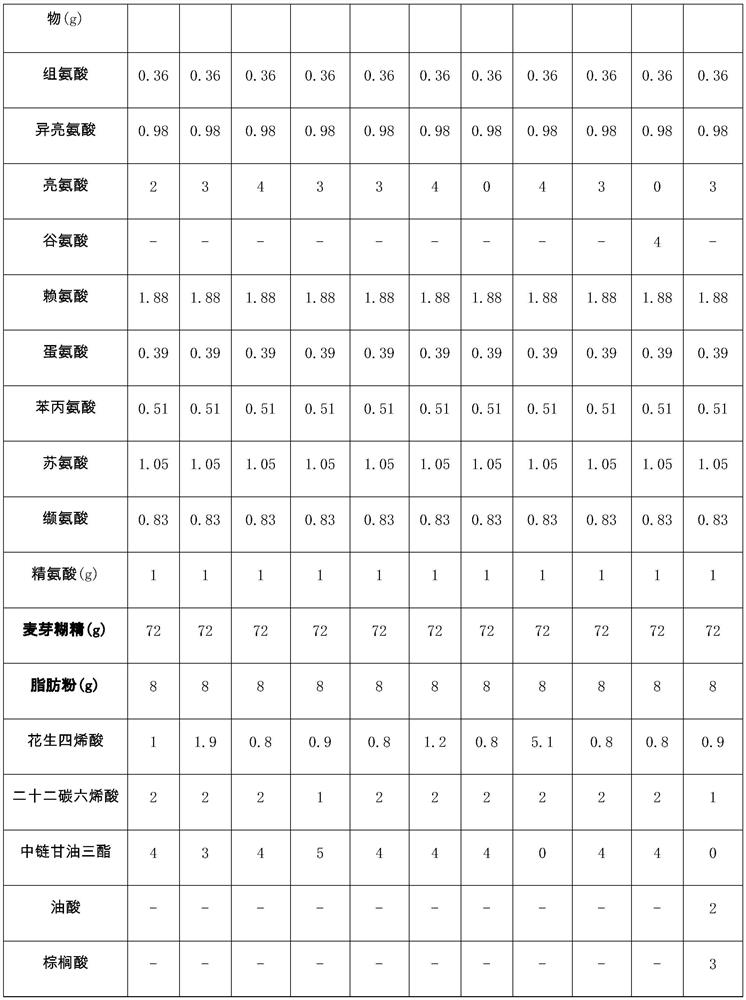
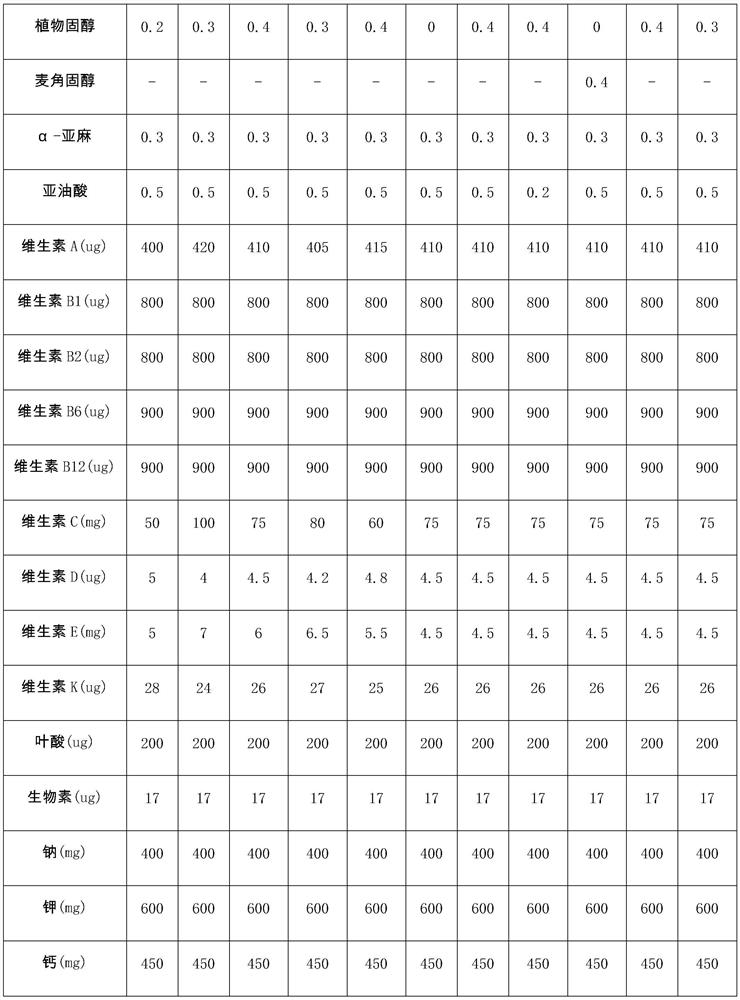
[0051] A composition suitable for patients with hyperlipidemic acute pancreatitis in the recovery period, containing 20g of polypeptide powder (5g of hydrolyzed whey protein, 3g of soybean peptide, 3g of casein calcium peptide, 8g-0.36g of essential amino acid mixture acid, 0.98g isoleucine, 2g leucine, 1.88g lysine, 0.39g methionine, 0.51g phenylalanine, 1.05g threonine, 0.83g valine, 1g arginine), 72g carbohydrates, 8g fat powder (1g arachidonic acid, 2g docosahexaenoic acid, 4g medium chain triglycerides, 0.2g phytosterols, 0.3g α-linolenic acid, 0.5g linoleic acid) and micronutrients. Wherein, the micronutrient vitamin A 400ug, vitamin B 1 800ug, Vitamin B 2 800ug, Vitamin B 6 900ug, Vitamin B 12 900ug, vitamin C 50mg, vitamin D5ug, vitamin E 5mg, vitamin K 28ug, folic acid 200ug, biotin 17ug, sodium 400mg, potassium 600mg, calcium 450mg, iron 5.5mg, zinc 4.5mg.
[0052] The preparation method of above-mentioned composition, comprises the following steps:
[0053...
Embodiment 2
[0060]A composition suitable for patients with hyperlipidemic acute pancreatitis in the recovery period, containing 20g of polypeptide powder (4g of hydrolyzed whey protein, 3g of soybean peptide, 3g of casein calcium peptide, 9g-0.36g of essential amino acid mixture acid, 0.98g isoleucine, 3g leucine, 1.88g lysine, 0.39g methionine, 0.51g phenylalanine, 1.05g threonine, 0.83g valine, 1g arginine), 72g carbohydrates, 8g fat powder (1.9g arachidonic acid, 2g docosahexaenoic acid, 3g medium chain triglycerides, 0.3g phytosterols, 0.3g α-linolenic acid, 0.5g linoleic acid ) and micronutrients. Wherein, the micronutrient vitamin A420ug, vitamin B 1 800ug, Vitamin B 2 800ug, Vitamin B 6 900ug, Vitamin B 12 900ug, vitamin C100mg, vitamin D 4ug, vitamin E7mg, vitamin K 24ug, folic acid 200ug, biotin 17ug, sodium 400mg, potassium 600mg, calcium 450mg, iron 5.5mg, zinc 4.5mg.
[0061] The preparation method of above-mentioned composition, comprises the following steps:
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com