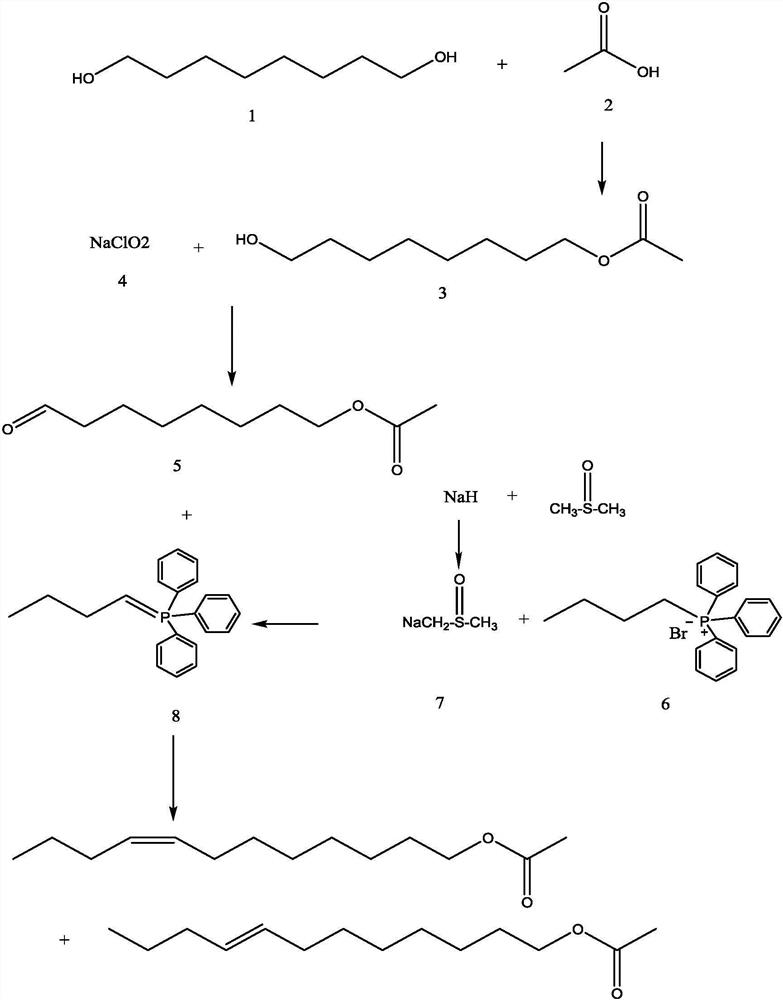
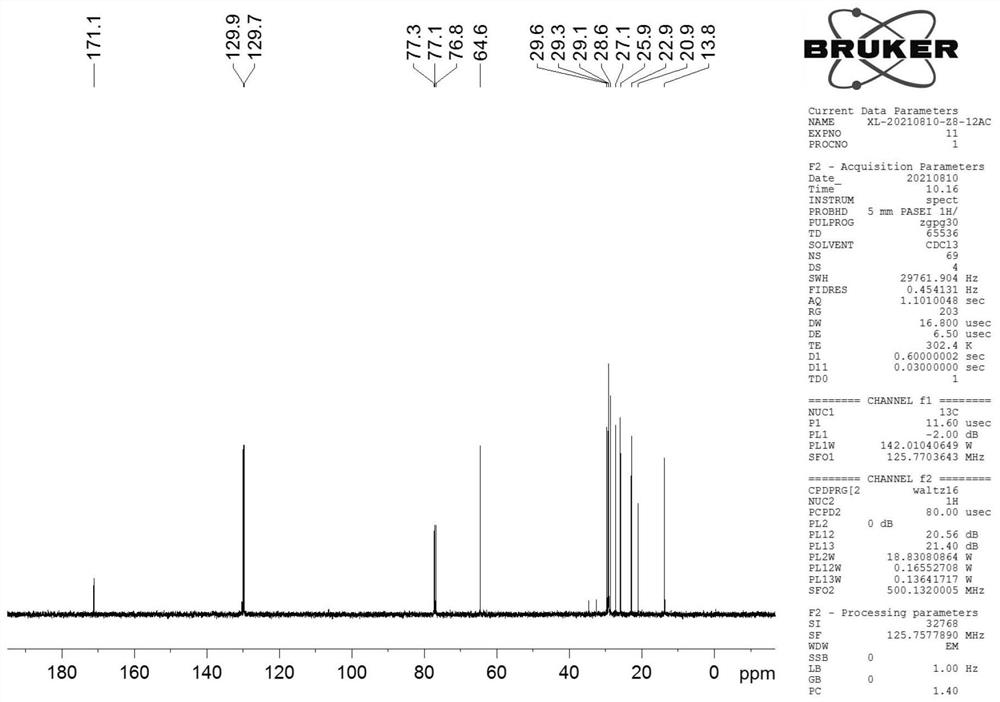
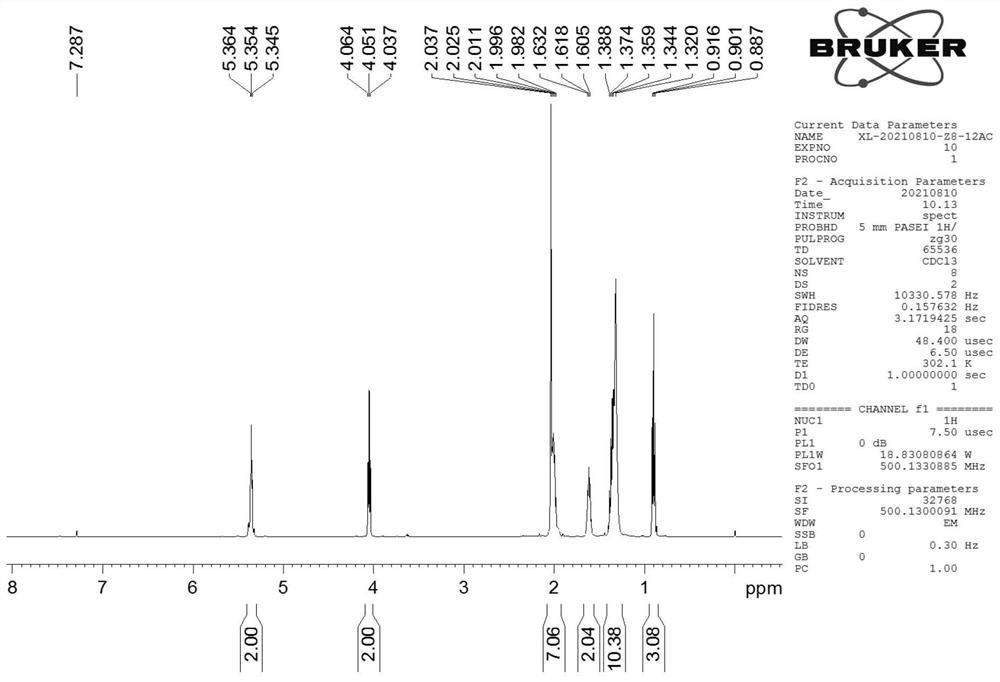
Synthesis method of cis (trans)-8-dodecenol acetate
A technology of carbenol acetate and synthesis method, which is applied in the field of artificial synthesis of insect sex pheromones, can solve the problems of high synthesis cost, difficult to obtain raw materials, cumbersome operation process, etc., achieve few steps, low production cost, and high production efficiency. The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of synthetic method of cis (trans)-8-dodecenol acetate, comprises the steps:
[0048] (1) At room temperature, place 1,8-octanediol in acetic acid aqueous solution, use sulfuric acid as a catalyst, and stir continuously to make 1,8-octanediol and acetic acid react to generate 8-acetoxy-1-octanol ; Use a continuous centrifugal extractor to continuously extract the 8-acetoxyl-1-octanol generated by the reaction with petroleum ether, after continuous extraction for 20 hours, obtain the petroleum ether phase, distill the petroleum ether in the petroleum ether phase, wait for the petroleum ether to evaporate After drying, 8-acetoxyl-1-octanol is obtained; the rotating speed of the continuous centrifugal extractor is 1450r / min, and the steamed petroleum ether continues to enter the continuous centrifugal extractor, and the petroleum ether is recycled and used;
[0049] (2) Add water, ethyl acetate, sodium bromide and sodium acetate trihydrate to 8-acetoxy-1-octanol, st...
Embodiment 2
[0070] The present embodiment is similar to the method of embodiment 1, and the difference between the two is as follows:
[0071] In step (1), the molar ratio of 1,8-octanediol, sulfuric acid and acetic acid is 1:0.5:5; in the acetic acid aqueous solution, the mass concentration of acetic acid is 25wt%;
[0072] In step (2), the molar ratio of 8-acetoxy-1-octanol, water, ethyl acetate, sodium bromide, sodium acetate trihydrate, tetramethylpiperidine oxide and sodium hypochlorite is 1:15: 10:0.5:0.5:0.005:1;
[0073] In step (3), in the preparation method of organic base: the molar ratio of sodium hydride, tetrahydrofuran and dimethyl sulfoxide is 1:1:2; in step (3), organic base, butyl triphenyl bromide The molar ratio of phosphine and 8-acetoxy-1-octanal is 2.5:1:1.
[0074] In this example, the yield of 8-acetoxy-1-octanol prepared in step (1) was 80%, and the yield of 8-acetoxy-1-octanal prepared in step (2) was 98%. %; The yield of the cis (trans)-8-dodecenol acetate p...
Embodiment 3
[0076] The present embodiment is similar to the method of embodiment 1, and the difference between the two is as follows:
[0077] In step (1), the extraction time is 10h;
[0078] In step (2-2), the temperature of the reaction system A is lowered to 0°C, the sodium hypochlorite solution is added dropwise, and the temperature of the reaction system A is controlled between 10°C, after the sodium hypochlorite solution is added dropwise, Insulated reaction at 10°C for 1 hour to obtain reaction system B;
[0079] In step (3-1) and step (3-2), the reaction temperature is controlled at -50°C.
[0080] In this example, the yield of 8-acetoxy-1-octanol prepared in step (1) was 75%, and the yield of 8-acetoxy-1-octanal prepared in step (2) was 94%. %; the yield of the cis (trans)-8-dodecenol acetate prepared by step (3) is 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com